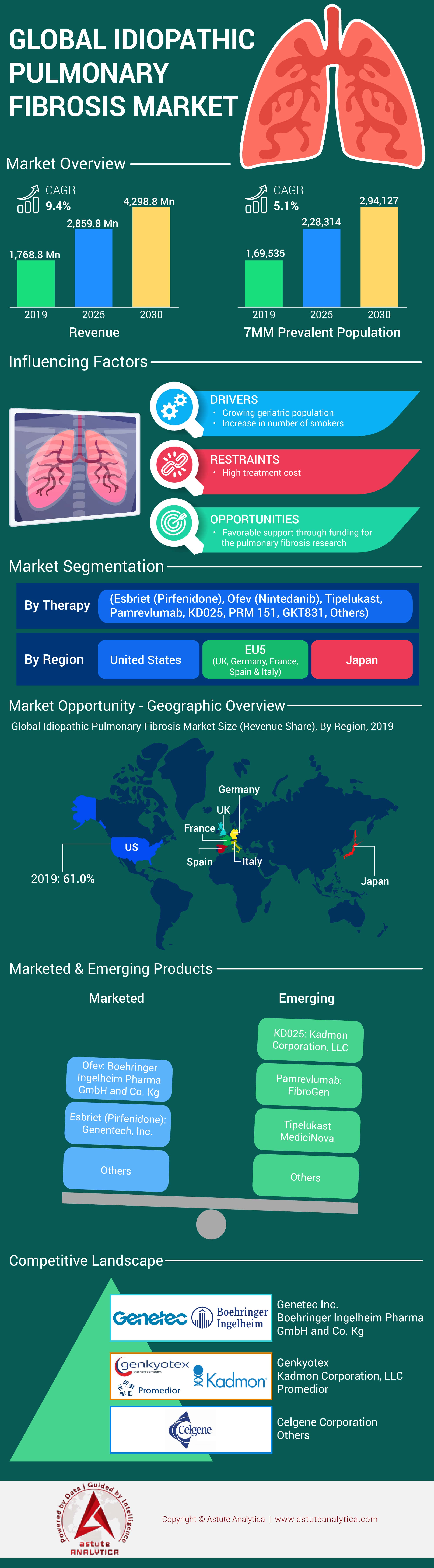
4.1. Idiopathic Pulmonary Fibrosis Overview
4.2. Industry Value Chain Analysis
4.2.1. Formulation
4.2.2. Processing & Packaging
4.2.3. Distributor
4.2.4. End users
4.3. Industry Outlook
4.3.1. Epidemiology and Patient Population
4.3.1.1. Key Findings
4.3.1.2. 7MM Prevalent Population of Idiopathic Pulmonary Fibrosis
4.3.1.3. Country Wise-Epidemiology of Idiopathic Pulmonary Fibrosis
4.3.1.3.1. United States
4.3.1.3.1.1. Prevalent cases of Idiopathic Pulmonary Fibrosis in the United States
4.3.1.3.1.2. Prevalent Population of Idiopathic Pulmonary Fibrosis by severity
4.3.1.3.1.3. Gender-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.1.4. Age-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2. EU5
4.3.1.3.2.1. Prevalent Population of Idiopathic Pulmonary Fibrosis in EU5
4.3.1.3.2.2. UK
4.3.1.3.2.2.1. Prevalent Population of Idiopathic Pulmonary Fibrosis
4.3.1.3.2.2.2. Prevalent Population of Idiopathic Pulmonary Fibrosis by severity
4.3.1.3.2.2.3. Gender-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.2.4. Age-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.3. France
4.3.1.3.2.3.1. Prevalent Population of Idiopathic Pulmonary Fibrosis
4.3.1.3.2.3.2. Prevalent Population of Idiopathic Pulmonary Fibrosis by severity
4.3.1.3.2.3.3. Gender-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.3.4. Age-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.4. Germany
4.3.1.3.2.4.1. Prevalent Population of Idiopathic Pulmonary Fibrosis
4.3.1.3.2.4.2. Prevalent Population of Idiopathic Pulmonary Fibrosis by severity
4.3.1.3.2.4.3. Gender-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.4.4. Age-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.5. Spain
4.3.1.3.2.5.1. Prevalent Population of Idiopathic Pulmonary Fibrosis
4.3.1.3.2.5.2. Prevalent Population of Idiopathic Pulmonary Fibrosis by severity
4.3.1.3.2.5.3. Gender-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.5.4. Age-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.6. Italy
4.3.1.3.2.6.1. Prevalent Population of Idiopathic Pulmonary Fibrosis
4.3.1.3.2.6.2. Prevalent Population of Idiopathic Pulmonary Fibrosis by severity
4.3.1.3.2.6.3. Gender-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.2.6.4. Age-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.3. Japan
4.3.1.3.3.1. Prevalent Population of Idiopathic Pulmonary Fibrosis
4.3.1.3.3.2. Prevalent Population of Idiopathic Pulmonary Fibrosis by severity
4.3.1.3.3.3. Gender-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.1.3.3.4. Age-specific Idiopathic Pulmonary Fibrosis Prevalence
4.3.2. Current Treatment Practices
4.3.2.1. ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis (An Update of 2011 Clinical Practice Guideline)
4.3.2.2. Patient Journey
4.3.3. Unmet Needs
4.3.4. Organizations contributing toward IPF
4.3.5. KOL's Views: Idiopathic Pulmonary Fibrosis
4.3.6. Case Reports
4.3.6.1. Idiopathic Pulmonary Fibrosis: As case Discussion in the US
4.3.6.2. Occurrence of idiopathic pulmonary fibrosis during immunosuppressive treatment: A Case Report of Europe
4.3.6.3. Nintedanib prevented fibrosis progression and lung cancer growth in idiopathic pulmonary fibrosis - A Japanese Case Report
4.3.7. Marketed Drugs
4.3.7.1. Esbriet (Pirfenidone): Inter Mune Inc.
4.3.7.1.1. Product Description
4.3.7.1.2. Regulatory Milestones
4.3.7.1.3. Clinical Development
4.3.7.1.4. Ongoing Current Pipeline Activity
4.3.7.1.5. Safety and efficacy
4.3.7.1.6. Product Profile
4.3.7.2. OFEV (Nintedanib): BoehringerIngelheim Pharma GmbH and Co. KG
4.3.7.2.1. Regulatory Milestones
4.3.7.2.2. Clinical Development
4.3.7.2.3. Ongoing Current Pipeline Activity
4.3.7.2.4. Safety and efficacy
4.3.7.2.5. Product Profile
4.3.8. Emerging Drugs
4.3.8.1. Tipelukast: MediciNova
4.3.8.1.1. Product Description
4.3.8.1.2. Other Development Activities
4.3.8.1.3. Clinical Development
4.3.8.1.4. Clinical Trials Information
4.3.8.1.5. Safety and Efficacy
4.3.8.1.6. Product Profile
4.3.8.2. Pamrevlumab: FibroGen
4.3.8.2.1. Product Description
4.3.8.2.2. Other Development Activities
4.3.8.2.3. Clinical Development
4.3.8.2.4. Clinical Trials Information
4.3.8.2.5. Safety and Efficacy
4.3.8.2.6. Product Profile
4.3.8.3. KD025: Kadmon Corporation, LLCs
4.3.8.3.1. Product Description
4.3.8.3.2. Other Development Activities
4.3.8.3.3. Clinical Development
4.3.8.3.4. Clinical Trials Information
4.3.8.3.5. Safety and Efficacy
4.3.8.3.6. Product Profile
(**to be continued in final report)
4.4. PESTLE Analysis
4.5. Porter's Five Forces Analysis
4.5.1. Bargaining Power of Suppliers
4.5.2. Bargaining Power of Buyers
4.5.3. Threat of Substitutes
4.5.4. Threat of New Entrants
4.5.5. Degree of Competition
4.6. Market Dynamics and Trends
4.6.1. Growth Drivers
4.6.2. Restraints
4.6.3. Challenges
4.6.4. Key Trends
4.7. Covid-19 Impact Assessment on Market Growth Trend
4.8. Market Growth and Outlook
4.8.1. Market Revenue Estimates and Forecast (US$ Mn), 2017 – 2030
4.8.2. Price Trend Analysis
4.9. Competition Dashboard
4.9.1. Market Concentration Rate
4.9.2. Company Market Share Analysis (Value %), 2020
4.9.3. Competitor Mapping
![pdf]()
![powerpoint]()
![excel]() | Report ID: AA0721084 | Delivery: 2 to 4 Hours
| Report ID: AA0721084 | Delivery: 2 to 4 Hours 

 | Report ID: AA0721084 | Delivery: 2 to 4 Hours
| Report ID: AA0721084 | Delivery: 2 to 4 Hours