Stem Cell Therapy Market: By Cell Source (Adipose tissue-derived MSCs (mesenchymal stem cells), Bone marrow-derived MSCs, Placental and umbilical cord-derived MSCs, Other Cell Sources); Type (Autologous stem cell therapy and Allogeneic Stem Cell Therapy); Delivery Method (In Vivo and Ex Vivo); Therapeutic Use (Oncology, Musculoskeletal disorders, Wounds and injuries, Cardiovascular, System diseases, Surgery, Inflammatory and autoimmune diseases, Nervous system disorders, Drug Discovery, Others); End Users (Hospitals, Ambulatory Surgical Centers, Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others); Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2025–2033
- Last Updated: Feb-2025 | Format:
![pdf]()
![powerpoint]()
![excel]() | Report ID: AA1224993 | Delivery: Immediate Access
| Report ID: AA1224993 | Delivery: Immediate Access
Market Scenario
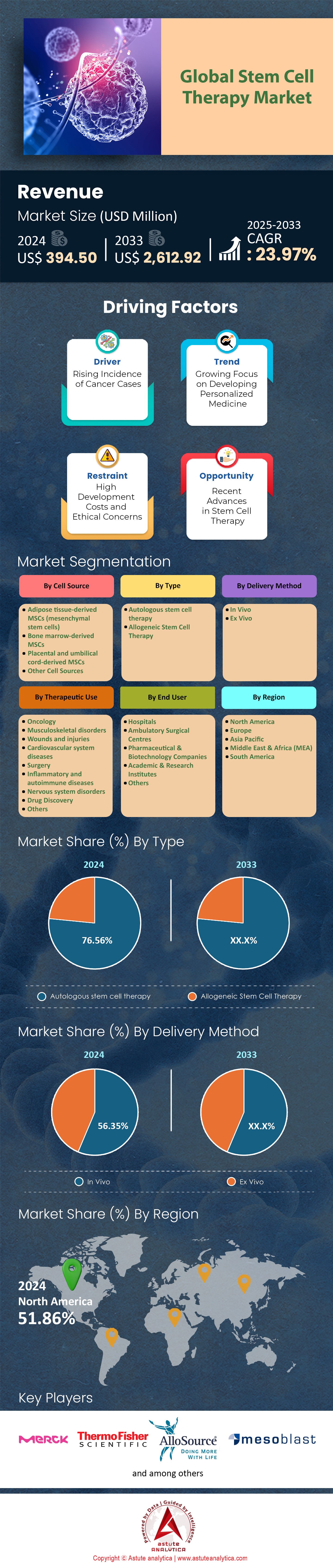
The stem cell therapy market is poised for remarkable growth, with revenues projected to rise from USD 394.50 million in 2024 to USD 2,612.92 million by 2033, reflecting a robust CAGR of 23.97% over the forecast period. This expansion is driven by factors such as the increasing prevalence of cancer, which is spurring demand for innovative treatments like stem cell therapies. Additionally, heightened investments in R&D and growing healthcare expenditure in key markets are accelerating advancements in stem cell technologies and broadening their therapeutic applications. In May 2020, the CiRA Foundation and CGT Catapult initiated a collaborative research project focused on the characterization of induced pluripotent stem cells. The partnership aims to leverage their combined expertise to explore innovative approaches for characterizing pluripotent stem cells, enhancing their use in the production of regenerative medicine products.
The growing shift toward personalized medicine, emphasizing treatments tailored to individual genetic profiles, is a key driver of market expansion. Stem cell therapy has emerged as a cornerstone of regenerative and targeted medicine, offering patient-specific solutions that address diverse medical needs. This precision-focused approach is expected to boost demand for stem cell-based therapies, opening new opportunities for market players and broadening their applications across various diseases. Leading institutions like the Wake Forest Institute for Regenerative Medicine are driving innovation, achieving milestones such as lab-grown organs, including bladders and tracheas, to alleviate organ shortages. Additionally, stem cell technologies are transforming drug discovery by providing human-relevant models that enhance the predictive accuracy of preclinical testing. These advancements underscore the transformative role of stem cells in personalized medicine, spanning tissue repair, tailored therapies, and next-generation drug development.
Moreover, researchers increasingly focused on developing stem cell therapies to combat COVID-19. For example, in January 2020, researchers from the University of Miami administered two stem cell infusions to COVID-19 patients suffering from lung damage. The study found the therapy to be reliable with no significant side effects.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Rising Incidence of Cancer Cases
The growing burden of cancer worldwide is significantly influencing the demand for advanced therapeutic solutions, particularly in the field of stem cell therapy. The rising global cancer burden is driving the demand for advanced therapeutic solutions, particularly in stem cell therapy. According to 2022 estimates from the International Agency for Research on Cancer (IARC), cancer cases continue to increase, disproportionately affecting underserved communities and highlighting the urgent need to address healthcare disparities. In 2022 alone, approximately 20 million new cancer cases were reported, along with 9.7 million cancer-related deaths. The lifetime risk of developing cancer is estimated at 1 in 5, with 1 in 9 men and 1 in 12 women succumbing to the disease. Among various cancer types, lung cancer remained the most prevalent, accounting for 2.5 million new cases (12.4%), followed by breast cancer (2.3 million, 11.6%), colorectal cancer (1.9 million, 9.6%), prostate cancer (1.5 million, 7.3%), and stomach cancer (970,000, 4.9%).
Further, The American Cancer Society's Global Cancer Statistics 2024 report projects that by 2050, cancer cases could rise to 35 million globally, driven by population growth and aging, if current incidence rates remain unchanged. The increasing cancer burden is a significant catalyst for the growth of the stem cell therapy market. These therapies provide innovative treatments for cancers such as leukemia, lymphoma, and multiple myeloma. For instance, Omisirge, developed by Gamida Cell, is an advanced cell therapy approved by the FDA in April 2023 for patients aged 12 and older with hematologic malignancies undergoing umbilical cord blood transplantation. As of the third quarter of 2023, the company reported the delivery of two Omisirge units and anticipated delivering a total of four to six units by the end of the year. By March 2024, Gamida Cell had delivered six units and successfully boarded 17 transplant centers, surpassing their initial target of 10 to 15 centers. The company has also secured confirmed coverage with U.S. payers covering more than 90% of commercial lives, exceeding their full-year goal of 70% (including confirmed coverage from all top 20 commercial payers alongside CMS)
Hematopoietic Stem Cell Transplantation (HSCT) is particularly notable, as it restores blood-forming cells destroyed during chemotherapy or radiation. The demand for advanced therapeutic solutions continues to spur research and development in stem cell therapies.
Restraint: High Development Costs and Ethical Concerns
One of the major challenges limiting the widespread adoption of stem cell therapy is its high treatment cost, making it inaccessible for many patients. In the United States, the average cost of stem cell therapy ranges between $5,000 and $50,000, with expenses often exceeding $50,000 for patients with advanced disease progression. These high costs pose a significant financial burden on patients, particularly in countries where insurance coverage for stem cell treatments is limited. As a result, affordability remains a critical barrier, slowing market growth and restricting access to potentially life-saving therapies. Additionally, clinical trials for cell-based therapies, spanning Phases I, II, and III, necessitate extensive patient recruitment, monitoring, and data collection, further driving up costs.
Ethical issues in stem cell research further complicate its progress. Ensuring that biological materials are donated voluntarily and with informed consent is a fundamental requirement. Research involving human embryonic stem cells (hESCs) raises continuous debates about the destruction of embryos, the creation of embryos specifically for research, and ethical concerns related to oocyte donation, such as payment, medical risks during retrieval, and protecting the reproductive rights of women in infertility treatments. The use of stem cell lines derived at other institutions often involves navigating conflicting legal and ethical standards.
Further, strict regulations in many countries govern the use of embryonic stem cells, with particular focus on the destruction of embryos and the implications of manipulating human cells at early developmental stages. Regulations on embryonic stem cell research vary globally. The UK enforces a 14-day limit with oversight from the HFEA, while Germany’s Embryo Protection Act strictly bans embryo creation for research. However, the Chinese government allows research on human embryos and cloning to continue for therapeutic purposes. Together, the significant financial demands, regulatory hurdles, and complex ethical landscape continue to challenge the pace of innovation and progress in the stem cell therapy market.
Opportunity: Recent Advances in Stem Cell Therapy
Recent advancements in stem cell therapies are revolutionizing the treatment of hard-to-treat diseases and transforming the manufacturing of advanced therapy medicinal products (ATMPs). In 2023, breakthroughs underscored the regenerative potential of stem cells, known for their ability to self-renew and differentiate into various tissue types. Although the first stem cell therapy was developed in 1957, only a few have been approved as ATMPs. This trend is changing rapidly, driven by technological innovations and evolving regulatory frameworks, such as the European Medicines Agency’s approval of a stem cell therapy Holoclar in 2015, which paved the way for accelerated development in the field.
One key breakthrough is in HIV treatment, where a March 2023 study (NCT02140944) demonstrated that cord blood stem cells successfully put the first mixed-race female patient into remission. This approach, unlike previous methods relying on adult donor cells, enhances the potential for a more inclusive and effective cure for HIV. Similarly, in February 2023, Journal of the American College of Cardiology reported that DREAM HF-1 trial for chronic heart failure found that mesenchymal precursor cells (MPCs) can greatly reduce inflammation, a major cause of heart failure. In patients with high inflammation, this treatment lowered the risk of heart attack or stroke by up to 75%. These findings highlight the growing potential of stem cell therapy beyond its mainstay cancer.
Advancements in clinical trials further illustrate the therapeutic potential of stem cells. For example, NCBI states that in Alzheimer’s disease, Phase I studies (NCT01297218/NCT01696591) have demonstrated that injecting Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSC) into the hippocampus and precuneus is safe and feasible, while another trial in Phase II (NCT02054208/NCT03172117) confirmed the safety of administering stem cells into the lateral ventricle via an Ommaya reservoir. Similarly, for Parkinson’s disease, the clinical trial (NCT03550183) is assessing the safety and efficacy of hUCB-MSC transplantation, with early results suggesting neuroprotection and functional improvement. These advancements highlight stem cell therapy’s growing potential in neurology, driving further research and commercialization.
Further, the advancement of gene-editing tools like CRISPR-Cas9 plays a pivotal role in enhancing the precision and safety of stem-cell therapies by enabling targeted genetic modifications. CRISPR-Cas9 has been employed in several instances to allow for fluorescent screening of developmental markers within a stem cell population. Researchers used CRISPR-Cas9 to create a COL2A1-GFP reporter iPSC line to differentiate and simultaneously purify chondrogenic cells. Furthermore, one of the biggest successes in recent times has been the CTX001, a CRISPR based therapy that was tested in clinical trials for sickle cell anemia and beta thalassemia treatment. Patient data reported at the ASH 2020 meeting indicated that promising results have been exhibited in 10 patients. The therapy was successful in restoring hemoglobin levels and alleviating pain bouts in all these patients. More significantly, the patients have gone several months without transfusion.
CRISPR-Cas9 has been explored as a potential HIV therapy by disrupting the CCR5 gene in hematopoietic stem cells (HSCs), creating HIV-resistant immune cells. Beyond HIV, ex vivo CRISPR editing of HSCs has been investigated for conditions like retinoblastoma, Werner syndrome, and chronic granulomatous disease. Moreover, the synergy between stem cell therapy and bioengineering is advancing functional tissue and organ development, integrating precision medicine, gene editing, and biomaterials to enhance regenerative treatments.
Segmental Analysis
Cell Source
Based on cell source, the adipose tissue-derived mesenchymal stem cells (ADSCs) segment is set to dominate the market, with a projected share of 40.41% in 2024, and is expected to achieve the highest CAGR through 2033. This growth is primarily driven by the ease in extraction of adipose tissue, which is rich in mesenchymal stem cells and can be harvested through minimally invasive procedures like liposuction. Compared to other sources, such as bone marrow, this ease of extraction reduces procedural complexity and costs, significantly boosting the segment's appeal.
ADSCs are widely utilized in regenerative medicine, particularly for musculoskeletal conditions and tissue repair. Studies have shown that injecting autologous adipose-derived stromal vascular fraction (ADSVFs) enriched with ADSCs into injured tendons improves their mechanical properties, increasing yield loads and energy absorption. Local ADSC injections have also enhanced muscle function, tendon healing, and reduced fatty infiltration in chronic rotator cuff tears, while boosting collagen I and III expression after Achilles tendon rupture. Their regenerative capabilities make them highly effective in treating bone and cartilage damage, such as osteoarthritis and traumatic injuries. Additionally, their anti-inflammatory properties enhance healing and tissue regeneration, further increasing their use in clinical trials and therapeutic applications.
Moreover, ADSCs are frequently used in autologous treatments, where the stem cells are derived from the same patient receiving the therapy. This approach minimizes the risk of immune rejection, offering a key advantage in treating disorders such as autoimmune diseases, cardiovascular conditions, and neurological issues. For example, RESTORE-2, the first clinical trial using ADRC-enriched fat grafting for breast reconstruction after BCT, demonstrated promising results, with 50 out of 67 patients reporting satisfaction over 12 months and no local cancer recurrence. With ongoing advancements in research, ADSCs are expected to expand into even more therapeutic areas, driving their rapid market growth and increasing adoption in diverse medical applications.
Additionally, bone marrow-derived MSCs hold 31.43% of the market, widely used in orthopedic and hematological disorders, though their invasive extraction process and lower cell yield limit their growth. Placental and umbilical cord-derived MSCs (18.04%) are gaining traction for their non-invasive collection, high proliferation capacity, and lower immune rejection risks, especially in neonatal and neurological treatments. The remaining 10.12% includes MSCs from sources like dental pulp, peripheral blood, and iPSCs, with potential for disease modeling and personalized therapies.
Type Insights
With the autologous segment retaining the biggest market share which is expected to cover more than half of the market in 2024 the worldwide stem cell therapy market is expanding significantly. By lowering the possibility of immunological rejection, this segment mostly uses stem cells that are produced from the patient's own body, which is a safer option than allogeneic (donor-derived) stem cells. Autologous stem cells are frequently employed in regenerative medicine for clinical purposes, particularly in the treatment of heart disease, autoimmune disorders, and orthopedic injuries.
Further, because of improvements in stem cell extraction, culture methods, and the growing number of approved treatments, the autologous market is also seeing the highest CAGR. The increasing need for customized medicine, wherein therapies employing a patient's own cells are thought to be more successful and have fewer adverse effects, is the main driver of this increase. Notably, autologous chondrocyte implantation (ACI) is a cell therapy for repairing full-thickness cartilage defects. It involves extracting, expanding, and reimplanting chondrocytes into the damaged area. Compared to microfracture, ACI offers similar short-term outcomes but superior long-term structural repair, with studies confirming its durability for up to 20 years. It has become routine treatment in some countries like United, States, Germany and UK.
Therapeutic Use Insights
The global stem cell therapy market is on a strong growth trajectory, with oncology emerging as the dominant segment, capturing 25.68% of the market share in 2024. The increasing application of stem cell therapies in cancer treatment is revolutionizing personalized medicine and enhancing patient outcomes. These therapies play a crucial role in regenerative medicine by repairing damaged tissues, particularly after chemotherapy or radiation. Notably, hematopoietic stem cell transplantation has become a cornerstone treatment for hematologic cancers like leukemia, restoring blood cell production in patients following intensive cancer therapies.
Meanwhile, the drug discovery segment is experiencing the most rapid expansion, boasting a remarkable CAGR. Stem cells are transforming pharmaceutical research by enabling more precise disease modeling in preclinical studies. Their ability to replicate human disease mechanisms in vitro enhances drug candidate identification, particularly for complex conditions like Alzheimer’s and Parkinson’s. By offering a more accurate biological representation than traditional cell lines, stem cells are accelerating the development of next-generation therapeutics. Supporting this progress, ClinicalTrials.gov reports 99 Phase III stem cell therapy trials that are either active or completed for various indications, highlighting their growing role in advanced treatment development.
End User Insights
The global stem cell therapy market is experiencing robust expansion, with the hospital segment emerging as the dominant player, projected to account for 50.74% of the market share in 2024. This leadership is driven by the growing adoption of stem cell therapies in hospitals, given their efficacy in treating chronic diseases, injuries, and degenerative conditions. Hospitals provide a highly controlled, specialized environment for administering these treatments, making them the preferred setting for most stem cell-based procedures. The hospital segment is also expected to register the highest CAGR 2025 to 2033. This accelerated growth is fueled by continuous advancements in medical technologies, increased healthcare investments, and expanding stem cell research. Leading hospitals are making substantial investments in cutting-edge infrastructure and specialized expertise to integrate stem cell-based treatments into mainstream medical care.
Further, academic & research institutes play a crucial role in advancing stem cell applications through preclinical and clinical studies, collaborating with biotech and pharmaceutical companies to develop novel therapies. The "others" segment includes stem cell banks that store and supply stem cells for medical use, specialty clinics offering regenerative treatments, and laboratories ensuring quality control and large-scale manufacturing of stem cell products. Together, these segments drive the growth and accessibility of stem cell therapy across medical and research applications.
Delivery Method Insights
The global stem cell therapy market is segmented into two key categories, In Vivo and Ex Vivo, each demonstrating distinct growth trajectories and market dynamics. As of 2024, the In Vivo segment leads the market, accounting for approximately 56.35% of the total share. This dominance is driven by the increasing demand for direct, in-body therapeutic applications of stem cells. In Vivo therapies involve the direct injection or implantation of stem cells into patients to treat a wide range of conditions, including cancer, cardiovascular diseases, and neurological disorders. The approach is particularly prevalent in regenerative medicine, where stem cells facilitate tissue repair and regeneration within targeted organs.
Meanwhile, the Ex Vivo segment—where stem cells are extracted, cultured externally, and then reintroduced for therapeutic use—is poised for the fastest expansion, with a projected compound annual growth rate (CAGR) of 24.36%. This rapid growth is fueled by advancements in stem cell culturing technologies and the rising adoption of personalized medicine. Ex Vivo therapies are particularly significant in bone marrow transplants for leukemia and the treatment of autoimmune diseases. The Ex Vivo segment’s impressive growth rate underscores the increasing investment in cell therapy research and development, reflecting a market shift towards more precise, targeted, and minimally invasive treatment solutions.
To Understand More About this Research: Request A Free Sample
Regional Insights
The global stem cell therapy market is poised for substantial growth in the coming years, with North America maintaining its leadership position, projected to account for almost half of the market share in 2024. This dominance is primarily driven by the region’s advanced healthcare infrastructure, high healthcare expenditure, and increasing adoption of stem cell-based treatments. The United States plays a pivotal role in this growth, benefiting from a strong presence of leading biotechnology and pharmaceutical firms such as Allogene Therapeutics, Fate Therapeutics, and CRISPR Therapeutics dedicated to stem cell research and development, alongside a favorable regulatory landscape that supports innovation in regenerative medicine.
Meanwhile, the Asia Pacific region is expected to witness the highest compound annual growth rate (CAGR) of 25.03% during the forecast period. This rapid expansion is fueled by advancements in healthcare systems, rising investments in medical research, and a growing emphasis on regenerative medicine. Countries such as China and Japan are at the forefront, driving progress through substantial investments in stem cell technologies, clinical trials, and the commercialization of therapies. Additionally, the region's large population and increasing prevalence of chronic diseases create a strong demand for innovative, next-generation healthcare solutions like stem cell therapy.
Clinical Overview of Omisirge and Ryoncil
| Drugs | Approval Date | Approved Indications | Pivotal Trial | Safety | Efficacy |
| Omisirge | 17-Apr-23 | Hematologic malignancies | Study P0501 (NCT02730299) | Among 117 patients who received Omisirge for any disease, infusion reactions occurred in 47% of patients (Grade II/IV in 15%), aGvHD in 58% (Grade III-IV in 17%), cGvHD in 35% and graft failure in 3% |
|
| Ryoncil | 18-Dec-24 | SR-aGvHD in pediatric patients | MSB-GVHD001 (NCT02336230) | sAEs occurred in 65% including pyrexia (9%), respiratory failure (9%), pneumatosis intestinalis (7%) and staphylococcal bacteremia (<5%). (N=54) |
|
Recent Developments
- In December 2024, Mesoblast Limited has announced that the FDA has approved Ryoncil (remestemcel-L) as the first mesenchymal stromal cell (MSC) therapy in the United States. Ryoncil is now the only MSC therapy approved in the U.S. and the first treatment available for steroid-refractory acute graft-versus-host disease (SR-aGvHD) in children as young as two months, including adolescents and teenagers. Dr. Joanne Kurtzberg, a leading transplant physician at Duke University Medical Center, emphasized the significance of this approval, stating that Ryoncil offers a life-saving option for children suffering from this severe condition, providing hope for both patients and their families.
- In December 2023, Bio-Techne Corporation announced the submission of a Drug Master File (DMF) to the U.S. Food and Drug Administration (FDA) for its ExCellerate GMP iPSC Expansion Medium, Animal Free (CCM0036-GMP). This medium is designed to support the development and production of regenerative medicine and stem cell therapies. Optimized to promote the robust expansion and maintenance of stem cell cultures, it ensures enhanced consistency and reproducibility throughout the manufacturing process.
- In November 2024, UBC researchers, Genome BC, and STEMCELL Technologies have launched a collaboration to accelerate stem cell research and its application in disease treatment. The project focuses on using transcription factors, proteins that control cell development, to simplify and speed up the process of generating specific cell types, such as heart, brain, or liver cells. By identifying the optimal combinations of these factors, the team aims to reduce the time required for cell differentiation, potentially expediting the development of new therapies for various diseases.
- In October 2024, Aspen Neuroscience, Inc. has announced the expansion of its San Diego operations with a new 22,000 square foot facility dedicated to the GMP manufacturing of induced pluripotent stem cell (iPSC)-derived therapies. This facility, located near the company’s headquarters, will focus on producing ANPD001, an iPSC-derived cell therapy for Parkinson’s Disease, as part of the ASPIRO study. The expansion includes ISO-certified manufacturing suites, quality control labs, and additional space for future growth and automation. Aspen is also advancing the industrialization of autologous cell therapy manufacturing to increase throughput and streamline production processes.
Top Players in Stem Cell Therapy Market
- Thermo Fisher Scientific, Inc
- AlloSource
- STEMCELL Technologies, Inc.
- Merck KGaA
- Sartorius AG (CellGenix GmbH)
- PromoCell GmbH
- Takara Holdings, Inc.
- Lonza
- ATCC
- AcceGen
- Cell Applications, Inc.
- Bio-Techne
- Cellular Engineering Technologies
- Mesoblast Ltd
- Other Prominent Players
Market Segmentation Overview:
By Cell Source
- Adipose tissue-derived MSCs (mesenchymal stem cells)
- Bone marrow-derived MSCs
- Placental and umbilical cord-derived MSCs
- Other Cell Sources
By Type
- Autologous stem cell therapy
- Allogeneic Stem Cell Therapy
By Delivery Method
- In Vivo
- Ex Vivo
By Therapeutic Use
- Oncology
- Musculoskeletal disorders
- Wounds and injuries
- Cardiovascular system diseases
- Surgery
- Inflammatory and autoimmune diseases
- Nervous system disorders
- Drug Discovery
- Others
By End User
- Hospitals
- Ambulatory Surgical Centres
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Others
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- Saudi Arabia
- South Africa
- UAE
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
View Full Infographic
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST

 | Report ID: AA1224993 | Delivery: Immediate Access
| Report ID: AA1224993 | Delivery: Immediate Access 
.svg)






