Saudi Arabia Generic Pharmaceutical Products Market: By Type (Antibacterial, Antiviral, Anti-hypertensive, Anti-inflammatory, Others); Application (Central Nervous System Disorders, Respiratory Diseases, Hormones & Related Diseases, Gastrointestinal Diseases, Cardiovascular Diseases, Infectious Diseases, Cancer, Diabetes, Others); Route Administration (Oral, Injectable, Inhalable, and Others); End Users (Hospitals, Homecare, Specialty Clinics, and Others); Distribution Channel (Online Pharmacies, Retail Pharmacies, and Hospital Pharmacies)—Market Forecast and Analysis for 2024–2032
- Last Updated: Jun-2024 | Format:
![pdf]()
![powerpoint]()
![excel]() | Report ID: AA0723492 | Delivery: 2 to 4 Hours
| Report ID: AA0723492 | Delivery: 2 to 4 Hours
Market Scenario
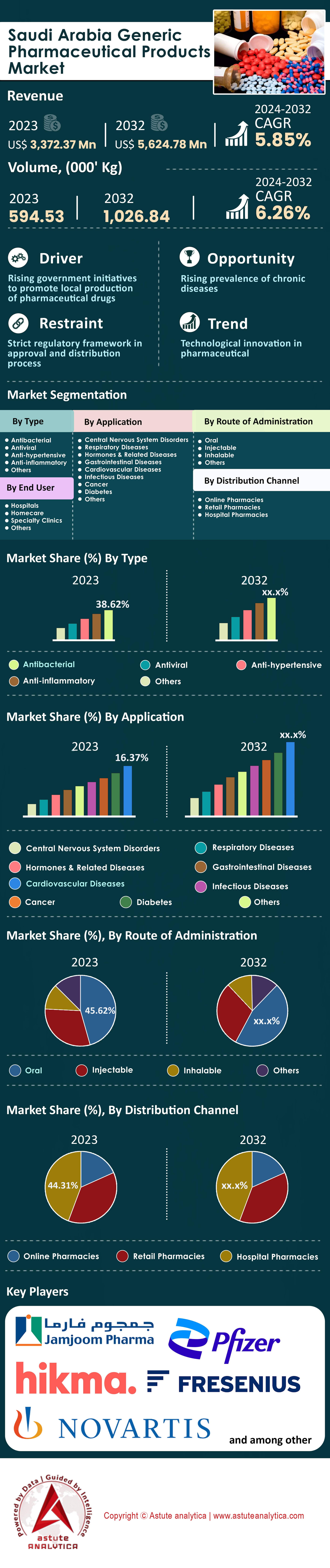
Saudi Arabia Generic Pharmaceutical Products Market was valued at US$ 3,372.37 million in 2023 and is projected to attain a valuation of US$ 5,624.78 million by 2032 at a CAGR of 5.85% During the Forecast Period 2024 to 2032.
For the past few years, Saudi Arabia has been witnessing increasing demand for cost-effective healthcare solutions. Generic drugs offer a more affordable alternative to branded medications, making them highly sought after by both consumers and healthcare providers. The government's focus on reducing healthcare expenses and improving access to quality healthcare is also fueling the market growth. Furthermore, the growing prevalence of chronic diseases, such as diabetes, cardiovascular disorders, and respiratory illnesses, is driving the demand for generic pharmaceutical products. According to a national survey conducted in Saudi Arabia, the estimated prevalence rates of chronic conditions, specifically diabetes mellitus (DM), were found to be 14.8% among males and 11.7% among females. As the population continues to age and lifestyle factors contribute to the rise in chronic conditions, the need for affordable and accessible medications will only increase.
The government of Saudi Arabia has been implementing various initiatives to encourage local production and reduce dependence on imported pharmaceutical products in the generic pharmaceutical products market. This strategy aims to enhance self-sufficiency and create a favorable environment for generic drug manufacturers. These initiatives, coupled with the streamlined regulatory framework, are expected to drive market growth by promoting domestic production and attracting foreign investments.
Nevertheless, the market also faces some challenges. One of the significant obstacles is the stringent regulatory requirements for generic drug approval. The process of gaining regulatory clearance can be time-consuming and expensive, which may deter some manufacturers from entering the market. Additionally, the presence of counterfeit and substandard drugs in the market poses a risk to patient safety and undermines the reputation of generic pharmaceutical products.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Increasing Government Initiatives and Support
The Saudi Arabian government has been actively promoting the local production of generic drugs and reducing the country's dependence on imported pharmaceutical products. These initiatives aim to enhance self-sufficiency, improve access to affordable medications, and stimulate the growth of the domestic pharmaceutical industry in the generic pharmaceutical products market.
The government has implemented policies that streamline the regulatory framework, making it more favorable for generic drug manufacturers. This includes expedited approval processes, simplified licensing procedures, and incentives for local production. Additionally, the government provides financial support and incentives for research and development in the pharmaceutical sector, encouraging innovation and the development of high-quality generic medications.
The government's focus on strengthening the healthcare sector and reducing healthcare expenses further drives the support for generic pharmaceutical products. With rising healthcare costs and the need for affordable treatment options, generic drugs offer a cost-effective solution, aligning with the government's goals of accessible and affordable healthcare for the population.
Trend: Increasing Focus on Biosimilars
Biosimilars are generic versions of biologic drugs that are highly similar in terms of quality, safety, and efficacy to the reference biologics. They offer cost savings and increased accessibility to expensive biologic treatments for various chronic diseases, such as rheumatoid arthritis, cancer, and diabetes in the Saud Arabia generic pharmaceutical products market.
The trend towards biosimilars is driven the patent expiration of many biologic drugs has opened opportunities for the development and commercialization of biosimilars. This allows for increased competition, leading to reduced prices and improved accessibility for patients.
Apart from this, the cost-effectiveness of biosimilars compared to their reference biologics is a significant factor in their adoption. Biosimilars offer substantial cost savings, which can alleviate the financial burden on healthcare systems and patients. This is particularly important in the treatment of chronic diseases, where long-term therapy is required. It has also been found that healthcare providers and regulatory authorities are becoming more confident in the safety and efficacy of biosimilars due to rigorous approval processes and increasing clinical evidence. As a result, there is a growing acceptance and utilization of biosimilars in the Saudi Arabian market.
Challenge: Increasing Penetration of Counterfeit and Substandard Drugs in the Market
A major challenge in the Saudi Arabia generic pharmaceutical products market is the presence of counterfeit and substandard drugs. Counterfeit drugs refer to those that intentionally misrepresent their identity or source, while substandard drugs are genuine drugs that fail to meet quality standards or specifications.
The existence of counterfeit and substandard drugs poses significant risks to patient safety and undermines the reputation of generic pharmaceutical products. Patients may unknowingly purchase and consume counterfeit or substandard drugs, leading to ineffective treatment outcomes, adverse reactions, or even fatalities.
Addressing this challenge in the Saudi Arabia generic pharmaceutical products market requires robust regulatory frameworks, strict enforcement, and increased vigilance. As a result, regulatory authorities and law enforcement agencies are collaborating to combat the production, distribution, and sale of counterfeit and substandard drugs. Strengthening supply chain integrity, implementing effective tracking and tracing systems, and raising public awareness about the risks of counterfeit drugs becoming essential in mitigating this challenge.
Additionally, promoting good manufacturing practices (GMP) and quality standards for generic pharmaceutical manufacturers has become of utmost importance. Thus, ensuring that all generic drugs in the market undergo rigorous quality testing and adhere to established standards. This, in turn, can help build trust among healthcare providers and patients.
The study suggests that efforts to tackle counterfeit and substandard drugs in the Saudi Arabia generic pharmaceutical products market should be multi-faceted, involving collaborations between government agencies, pharmaceutical companies, healthcare professionals, and public awareness campaigns. Continuous monitoring, stringent penalties for offenders, and regular inspections of manufacturing facilities and pharmacies are vital in maintaining the integrity of the Saudi Arabia generic pharmaceutical products
Segmental Analysis
By Type
In terms of product type, the Saudi Arabia generic pharmaceutical products market is primarily dominated by the Antibacterial segment. Antibacterial drugs accounted for the highest share of 38.62% in 2023, indicating their significance in the market. This segment is projected to maintain its dominance throughout the forecast period and exhibit the fastest compound annual growth rate (CAGR) of 6.27%.
The dominance of the Antibacterial segment can be attributed to several factors. Saudi Arabia, like many other countries, faces a considerable burden of infectious diseases, including bacterial infections. The high prevalence of bacterial infections, coupled with the need for cost-effective treatment options, drives the demand for generic Antibacterial drugs. These medications offer similar efficacy as branded counterparts but at a more affordable price point, making them a preferred choice for healthcare providers and patients.
By Application
Within the Saudi Arabia generic pharmaceutical products market, the cardiovascular diseases segment holds significant potential. It is projected to generate over 16.37% of the market's revenue in the coming years and exhibit healthy growth at a CAGR of 6.44% during the projection period from 2024 to 2032.
The prevalence of cardiovascular diseases, including hypertension, coronary artery disease, and heart failure, is on the rise in Saudi Arabia. According to data from the Saudi Ministry of Health, CVDs are the leading cause of mortality in Saudi Arabia, accounting for a substantial proportion of deaths. Reports indicate that approximately 40% of deaths in the country are attributed to CVDs. These statistics highlight the significant burden that cardiovascular diseases impose on the population.
The high prevalence of the diseases in the generic pharmaceutical products market is primarily attributed to sedentary lifestyles, unhealthy dietary habits, and an aging population contribute to the increased burden of cardiovascular disorders. As a result, there is a growing demand for cost-effective generic pharmaceutical products to manage these conditions.
Generic medications targeting cardiovascular diseases offer affordability without compromising on quality or efficacy. They provide healthcare practitioners and patients with accessible treatment options to manage and control cardiovascular conditions. The cost savings associated with generic drugs also make them favorable in long-term treatment regimens, where adherence to therapy is crucial.
By Route of Administration
The oral route of administration dominates the Saudi Arabia generic pharmaceutical products market, accounting for 45.62% of the market share in 2023. This segment is projected to continue its dominance and exhibit the highest CAGR of 6.20% over the forecast period from 2024 to 2032.
The preference for oral medications can be attributed to their ease of administration, convenience, and patient compliance. Oral generic pharmaceutical products are available in various forms such as tablets, capsules, and syrups, making them suitable for a wide range of patient populations, including children and elderly individuals. Additionally, the oral route offers a non-invasive mode of drug delivery, which is preferred by many patients.
The dominance of the oral route of administration in the Saudi Arabia generic pharmaceutical products market is also influenced by the types of therapeutic areas where generic drugs are commonly prescribed. Conditions such as chronic diseases, infections, and pain management often require long-term treatment, and oral medications are the most practical and cost-effective option for such cases. The ease of self-administration also adds to the popularity of oral generic pharmaceutical products.
To Understand More About this Research: Request A Free Sample
Recent Developments:
- 2021 January 14: The Kingdom of Saudi Arabia (KSA) put forth a fresh pricing policy, redefining the financial landscape of the local pharmaceutical industry. This new policy modified the criteria for pricing Generic and other medications in the country, and it also reduced the Kingdom’s reference basket from a tally of 30 countries down to just 20.
- 2021 December 7: Biocon Limited and Tabuk Pharmaceutical Manufacturing Company declared a collaborative venture with the shared goal to develop, manufacture, and commercialize Biocon's specialty Generic Pharmaceutical Medicines. This partnership is set to expand Biocon's influence not only in Saudi Arabia but also throughout the Middle East and North Africa (MENA) region.
- 2023 June 21: In a landmark financial event, Jamjoom Pharmaceuticals Factory Company announced a formidable US$ 336 million Initial Public Offering (IPO) on the Tadawul Stock Exchange in Saudi Arabia. This strategic move is intended to stimulate the growth of its Generic Pharmaceutical Products business not just within Saudi Arabia, but also across the Middle East and Africa (MEA) region.
Major Players in the Saudi Arabia Generic Pharmaceutical Products Market
- Advanced Pharmaceutical Factory
- AstraZeneca
- Fresenius SE & Co. KGaA
- Hikma Pharmaceuticals PLC
- Jamjoom Pharma
- Novartis AG
- Pfizer Inc.
- SAJA Pharmaceuticals
- Tabuk Pharmaceuticals
- Other Prominent Players
Market Segmentation Overview:
By Type
- Antibacterial
- Antiviral
- Anti-hypertensive
- Anti-inflammatory
- Others
By Application
- Central Nervous System Disorders
- Respiratory Diseases
- Hormones & Related Diseases
- Gastrointestinal Diseases
- Cardiovascular Diseases
- Infectious Diseases
- Cancer
- Diabetes
- Others
By Route Administration
- Oral
- Injectable
- Inhalable
- Others
By End Users
- Hospitals
- Homecare
- Specialty Clinics
- Others
By Distribution Channel
- Online Pharmacies
- Retail Pharmacies
- Hospital Pharmacies
View Full Infographic
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST

 | Report ID: AA0723492 | Delivery: 2 to 4 Hours
| Report ID: AA0723492 | Delivery: 2 to 4 Hours 
.svg)






