Neurostimulation Devices Market: By Type (Implantable Device and External Device); Application (Pain Management, Hearing Loss, Urinary Incontinence, Parkinson's Disease, and Others); End User (Hospitals/Clinics, Cognitive Care Centers, Research Institutes, and Others); and Region—Industry Dynamics, Market Size and Opportunity Forecast for 2025–2033
- Last Updated: 30-Jan-2025 | | Report ID: AA0522231
Market Snapshot
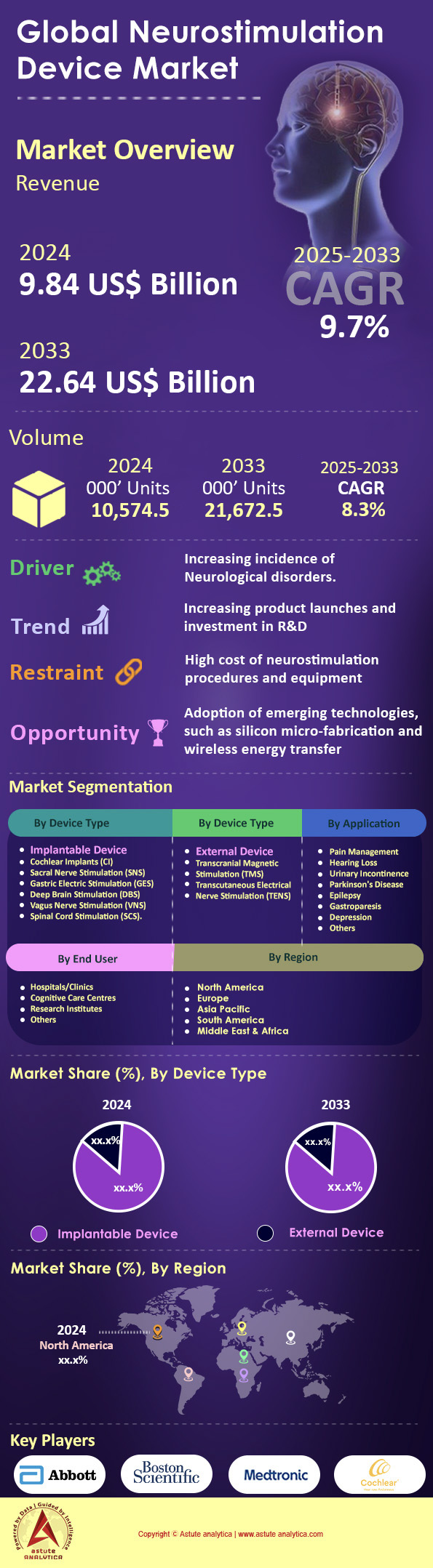
Neurostimulation devices market was valued at US$ 9.84 billion in 2024 and is projected to hit the market valuation of US$ 22.64 billion by 2033 at a CAGR of 9.7% during the forecast period 2025–2033.
The neurostimulation devices market is poised for remarkable growth, driven by increasing clinical applications, technological maturity, and expanding patient populations. Across multiple therapeutic areas—ranging from chronic pain management to psychiatric disorders—these devices offer a promising avenue for individuals unresponsive to traditional treatments. As of May 2024, there are 3,388 Alzheimer’s disease clinical trials underway, many of which include investigational neurostimulation therapies. Furthermore, the United States alone accounts for a substantial portion of ongoing research, as ClinicalTrials.gov lists 524,411 registered studies overall as of January 2025, reflecting an immense scope of clinical exploration.
This surge in clinical attention coincides with the reality that an estimated 80 million people in the U.S. suffer from chronic pain, indicating a pressing need for more innovative solutions. Traditional interventions in the neurostimulation devices market remain limited: only around 3 out of 10 individuals with non-cancer chronic pain experience significant relief from standard therapies, underlining the critical role of neurostimulation. Leading healthcare institutions such as the Mayo Clinic already treat over 1,700 people annually with deep brain stimulation (DBS), suggesting a high and growing demand for these approaches. Regulatory bodies have also kept pace with market needs. For instance, the Medtronic Percept™ RC Deep Brain Stimulation system received FDA approval in January 2024, expanding the variety of neurostimulation offerings. Alongside Parkinson’s disease, DBS is now being investigated for multiple neurological conditions, reflecting the market’s wide-ranging future potential.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Increasing acceptance of targeted neuromodulation for refractory ailments across specialized healthcare clinical settings worldwide
Clinicians are adopting sophisticated neurostimulation therapies to tackle conditions that fail to respond to conventional treatments. A neurology center in Denmark neurostimulation devices market tested 2 new stimulation paradigms for fibromyalgia in 2023, revealing broader possibilities for pain management. A hospital in Boston documented average daily usage of 18 hours for implantable TENS devices among individuals with severe neuropathy unresponsive to medications. Researchers in Toronto have pursued advanced dorsal root ganglion stimulation with 7 directional leads to boost localized analgesia. These developments signal a growing acceptance that neuromodulation can significantly extend or replace standard care protocols.
Another dimension fueling adoption across the neurostimulation devices market is the evolving ecosystem of real-time data insights. An observational study in 2024 reported up to 6 patient enrollments monthly for SCS trials targeting complex regional pain syndrome. Physicians in Israel implemented closed-loop vagus nerve stimulation in an epilepsy program, maintaining 3 consecutive months of improved seizure control in drug-resistant cases. An FDA-cleared wearable TMS device from Cervel Neurotech shortened acute depression therapy sessions by 20 minutes, meeting the increasing demand for adaptable, patient-friendly solutions. Such initiatives reflect how specialized healthcare providers regard targeted neuromodulation as a practical option to achieve measurable clinical improvements.
Trend: Progressive shift toward closed-loop systems with real-time biofeedback for optimized stimulation outcomes globally
Closed-loop neurostimulation has emerged as a major trend in the neurostimulation devices market, relying on continuous patient data to auto-adjust therapies. A motion-sensing spinal implant tested in Cambridge in 2024 modifies pulse amplitude within 2 milliseconds to match patient movements accurately. Researchers in Lyon introduced a deep brain stimulator with 5 integrated electrodes that respond to fluctuating motor signals in essential tremor. Meanwhile, a device from Boston Scientific’s research division captures neural feedback every 90 seconds to tailor waveforms dynamically for spinal cord pain relief. These refinements promise more precise interventions that monitor and correct stimulation settings in real time.
Enhanced analytics and connectivity also bolster the closed-loop trend. A clinical trial in Melbourne utilized cloud-based dashboards that interpret cortical signals from 2 invasive electrodes, allowing specialists to fine-tune TMS sessions on demand. One newly released system from Abbott logs usage patterns every hour for advanced analytics, highlighting where therapy adjustments are most needed. Neurologists in Seoul tested an implant that shifts from low-frequency to high-frequency output after detecting 5 successive abnormal pulses, demonstrating how agile programming can preempt symptom escalation. Together, these tools illustrate an ongoing push for more personalized and context-sensitive neuromodulation treatments designed to maximize efficacy and reduce side effects.
Challenge: Maintaining device biocompatibility and long-term performance under rigorous implant and usage conditions consistently
While impressive performance gains define modern neurostimulation, ensuring devices remain safe and effective under prolonged use is a formidable challenge in the neurostimulation devices market. Engineers at a medical technology lab in Singapore identified micro-fissures in titanium casings after 17 months of patient usage in high-humidity climates. Battery specialists in Houston discovered that rapid recharging protocols generate a temperature spike of 9 degrees Celsius, risking tissue irritation adjacent to the implant. Frequent firmware updates present added complexity, as one neurology consortium in Geneva found slight memory corruption in 2 older DBS models when introducing new software modules.
Clinicians and manufacturers in the neurostimulation devices market are pursuing advanced materials and rigorous testing to address these issues. A reliability study in Stockholm tested a polymer-insulated lead for 11 months to assess corrosion resistance under continuous fluid exposure. A research team in Tokyo improved heat dissipation by integrating graphene layers measuring less than 1 micron thick around battery cells. Meanwhile, neurosurgeons in São Paulo performed a long-term assessment, documenting less than 1 millimeter of electrode displacement over 14 months in patients engaged in daily physical therapy. Such measures reflect the ongoing quest to perfect device durability and limit complications in real-life settings.
Segmental Analysis
By Device Type
Implantable devices account for over 85% of the neurostimulation devices market due to their highly targeted therapeutic delivery, long-term treatment stability, and reduced external maintenance requirements. More than 4 million advanced implants have been performed worldwide in the last ten years, underscoring a rising preference for more permanent solutions to critical neurological disorders. An estimated 16 million individuals globally could benefit from such interventions as they grapple with Parkinson’s disease, epilepsy, severe hearing impairments, and neuropathic pain. Spinal cord stimulators, deep brain stimulators, sacral nerve stimulators, vagus nerve stimulators, and cochlear implants are among the most popular systems approved for implantation In clinical settings, over 600,000 new cases of complex brain or spinal pathway deficits arise annually, prompting specialized practitioners to explore implantable devices for more precise nerve modulation. Furthermore, research suggests at least 2 million new device recipients will be added in the next five years as implantation procedures become safer and more accessible.
This strong demand is also propelled by the potential to manage debilitating conditions that affect daily function. Over 18 million people worldwide live with some form of chronic neurological or sensory deficit that fails to respond to pharmaceuticals alone, making permanent implants a vital option for sustainable relief. Among the devices, deep brain stimulators have already surpassed 500,000 cumulative implants in the global neurostimulation devices market, exemplifying their pivotal role in Parkinson’s and essential tremor therapies. Meanwhile, spinal cord implants are nearing 750,000 total procedures, primarily for refractory chronic pain. Key end users of these solutions include multispecialty hospitals, neurology centers, and advanced rehabilitation clinics, where highly trained teams evaluate each patient’s candidacy for implant-based care. Given the growing geriatric demographic and an uptick in younger patients experiencing traumatic injuries, the global potential user population for such technology is on track to expand significantly, driven by the continuous evolution of clinical guidelines and patient awareness of implant benefits.
By Application
Pain management application generates over 39.40% of the global neurostimulation devices market because this approach offers targeted relief with fewer systemic side effects, particularly for patients who do not respond well to conventional medication. Reports indicate that around 1.4 billion individuals worldwide suffer from chronic pain, with at least 350 million enduring pain for longer than six months. Among those, over 200 million present with severe back pain tied to degenerative conditions or post-surgical complications, making them prime candidates for spinal cord stimulation. An additional 120 million people experience neuropathic pain linked to conditions such as diabetic neuropathy or postherpetic neuralgia, driving expansions in peripheral nerve and dorsal root ganglion stimulators. Annual estimates suggest that 500,000 new patients develop complex regional pain syndromes where drug-based strategies fail, bolstering clinician interest in fast-acting neurostimulation implants.
This strong demand for this application in the neurostimulation devices market is especially evident in populations struggling with acute pain episodes that escalate into chronic states. Approximately 70 million individuals worldwide develop migraine or cluster headaches yearly, often resistant to standard therapies. Meanwhile, over 250 million working adults report repetitive stress injuries that result in chronic musculoskeletal discomfort. Spinal cord stimulators, peripheral nerve stimulators, and intrathecal drug delivery pumps remain the key devices in pain management, offering adjustable intensity settings that cater to patient-specific thresholds. Clinics performing over 600,000 procedures annually consistently underscore neurostimulation’s efficacy in cutting back pain medication dosage by up to 50%, which helps mitigate opioid-related dependence. Deep brain stimulators, though often utilized for movement disorders, are also used in select chronic pain cases unresponsive to other methods. With a persistent increase in degenerative spinal conditions and complex pain disorders, the global population seeking neurostimulation for lasting pain relief is forecasted to climb, further solidifying the leading role of pain management within this market segment.
By End Users
Hospitals and clinics collectively capture more than 84.9% of the neurostimulation devices market because they offer integrated services that span diagnosis, implant surgery, and postoperative care. Each year, over 1.2 million neurosurgical and neurological procedures are conducted in major hospitals, with a sizable fraction involving implantable stimulators for pain relief, movement disorders, or functional rehabilitation. Around 85,000 specialized neurologists and neurosurgeons worldwide operate within these facilities, significantly influencing adoption rates. Over 400,000 new patients are funneled into hospital-based evaluation programs annually, where they undergo thorough imaging and electrophysiology analyses that confirm candidacy for device implantation. Additionally, an estimated 900,000 follow-up visits per year occur in the same hospital or clinic setting, ensuring consistent monitoring of each patient’s neural stimulation levels.
Multidisciplinary expertise at these institutions fosters higher procedural success rates and better long-term patient outcomes. Recent estimates in the neurostimulation devices market show that more than 70% of revision surgeries or re-implantations are performed by specialized hospital teams equipped with advanced radiological and surgical technologies. Meanwhile, rural and small-city hospitals account for over 300,000 outpatient visits where initial neuromodulation screening takes place, gradually routing complex cases to tertiary-care centers. Clinics with dedicated pain management units perform at least 40,000 spinal cord stimulator trials per year, enabling stepwise evaluations before permanent device placement. This continuum of care reassures patients, while close collaboration among anesthesiologists, neurologists, and physiotherapists streamlines the therapy path. Moreover, an estimated 25% growth in outpatient neurology clinics over the past decade has heightened awareness of neurostimulation among primary care clinicians, accelerating referrals. Because hospitals and clinics maintain this end-to-end ecosystem—covering preoperative counseling, state-of-the-art implantation, and extended rehabilitation—they remain the undisputed hub for neurostimulation device use, consistently reinforcing their dominant status in the global market.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
To Understand More About this Research: Request A Free Sample
Regional Analysis
North America exerts dominant control over the neurostimulation devices market, holding more than 41.7% of the global market share, largely due to a robust healthcare infrastructure and an extensive insurance framework that supports advanced medical procedures. Currently, over 25 million people in this region deal with chronic neurological conditions, many of which are amenable to neurostimulation. In the past seven years, more than 820,000 implant procedures have been completed in North American hospitals, reflecting a mature regulatory environment that speeds up clinical trials and device approvals. The region also has over 40 specialized neuromodulation research facilities, driving continuous product advancements. Each year, around 120,000 new candidates pass eligibility screenings for spinal cord or deep brain stimulators based on multidisciplinary evaluations. Furthermore, an estimated 65% of leading pain management clinics in Canada and the U.S. integrate neurostimulation into their protocols for refractory back pain, fibromyalgia, or neuropathic pain. At least 700 neurosurgeons nationwide specialize in advanced neuromodulation interventions, bolstering procedural volumes. Notably, around 600,000 outpatient visits per quarter are linked to follow-up sessions for implanted neurostimulators, further reinforcing the region’s systemic capacity to handle complex, device-based therapies.
Within North America neurostimulation devices market, the United States stands out as the single largest contributor, accounting for more than 70% of regional neurostimulation-related revenue. One key driver is the country’s vast base of approximately 280 million insured individuals, which makes high-end treatments more accessible. In addition, the U.S. exhibits an unparalleled density of device manufacturers, with many global frontrunners headquartered within its borders. Companies like Abbott and Boston Scientific regularly pilot new stimulators and implant techniques through large-scale clinical trials, influencing global best practices. Over 10,000 physiatrists, neurologists, and surgeons complete specialized training courses each year, expanding expertise in these solutions and steering patient referrals toward implant-based interventions. These factors collectively lead to accelerated adoption rates, driving overall market expansion. Consequently, North America remains the primary powerhouse for neurostimulation, setting both scientific and clinical benchmarks that other regions often emulate, and ensuring the region’s continued hegemony in this evolving field.
Top Companies in Neurostimulation Devices Market:
- Abbott Laboratories
- Advanced Bionics
- B. Braun Melsungen AG
- Boston Scientific Corporation
- BrainsWay
- Cochlear
- CONMED Corporation
- KONINKLIJKE PHILIPS
- Laborie Medical Technologies, Inc.
- MED-EL Medical Electronics
- Medtronic PLC
- Nevro Corp
- Zimmer Biomet Holdings, Inc.
- Other Prominent Players
Market Segmentation Overview:
By Device Type:
- Implantable Device
- Cochlear Implants (CI)
- Sacral Nerve Stimulation (SNS)
- Gastric Electric Stimulation (GES)
- Deep Brain Stimulation (DBS)
- Vagus Nerve Stimulation (VNS)
- Spinal Cord Stimulation (SCS)
- External Device
- Transcranial Magnetic Stimulation (TMS)
- Transcutaneous Electrical Nerve Stimulation (TENS)
By Application:
- Pain Management
- Hearing Loss
- Urinary Incontinence
- Parkinson's Disease
- Epilepsy
- Gastroparesis
- Depression
- Others
By End User:
- Hospitals/Clinics
- Cognitive Care Centres
- Research Institutes
- Others
By Region:
- North America
- The U.S.
- Canada
- Mexico
- Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Australia & New Zealand
- Rest of APAC
- Middle East & Africa (MEA)
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
REPORT SCOPE
| Report Attribute | Details |
|---|---|
| Market Size Value in 2024 | US$ 9.84 Bn |
| Expected Revenue in 2033 | US$ 22.64 Bn |
| Historic Data | 2020-2023 |
| Base Year | 2024 |
| Forecast Period | 2025-2033 |
| Unit | Value (USD Bn) |
| CAGR | 9.7% |
| Segments covered | By Device Type, By Application, By End-User, By Region |
| Key Companies | Abbott Laboratories, Advanced Bionics, B. Braun Melsungen AG, Boston Scientific Corporation, BrainsWay, Cochlear, CONMED Corporation, KONINKLIJKE PHILIPS, Laborie Medical Technologies, Inc., MED-EL Medical Electronics, Medtronic PLC, Nevro Corp, Zimmer Biomet Holdings, Inc., Other Prominent Players |
| Customization Scope | Get your customized report as per your preference. Ask for customization |
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)