Medical Devices Market By Product (Diagnostic Devices, Consumables, Patient Aids, Others); By Application (Oncology, Cardiology, Orthopedics, Ophthalmic, Respiratory, Others); By End-Users (Hospitals & Surgical Centres, Clinics, Household, Others); By Region (North America, Europe, Asia Pacific, South America, Middle East and Africa)- Opportunity Analysis and Forecast To 2031
- Last Updated: Jan-2025 | Format:
![pdf]()
![powerpoint]()
![excel]() | Report ID: AA1021097 | Delivery: 2 to 4 Hours
| Report ID: AA1021097 | Delivery: 2 to 4 Hours
Market Scenario
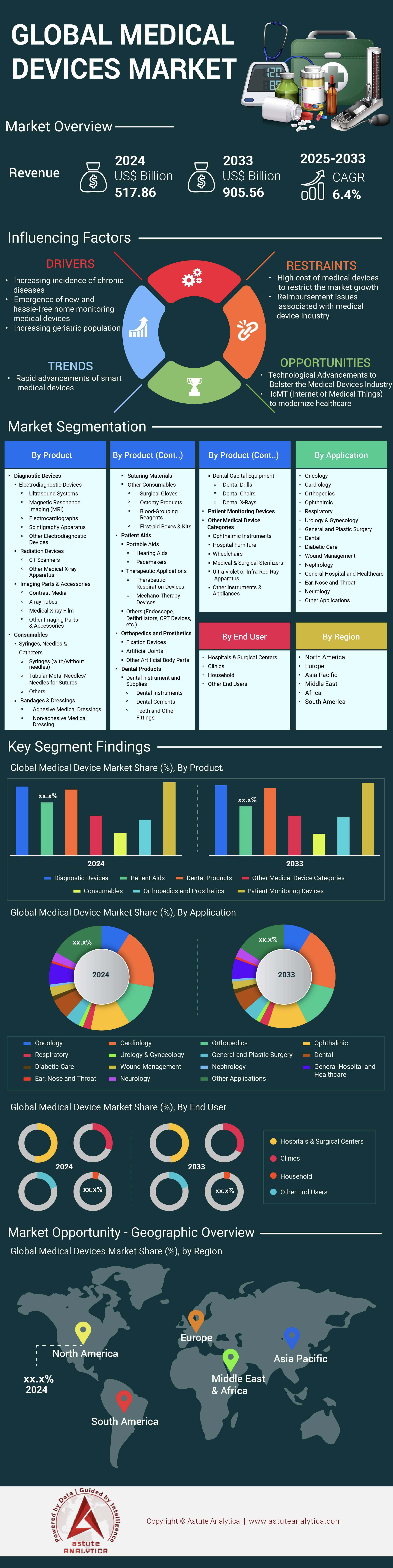
Medical devices market was valued at US$ 517.86 billion in 2024 and is projected to reach US$ 905.56 billion by 2033, growing at a CAGR of 6.4% during the Forecast Period 2025–2033.
The medical devices market continues to experience dynamic growth, driven by heightened emphasis on personalized care and early diagnostics. The global revenues are projected to surpass US$905 billion by 2033, reflecting robust investments in wearable sensors, robotic surgery systems, and advanced imaging tools. The proliferation of digitally connected devices is becoming ever more apparent, with an estimated 504 million wearable health monitors expected to ship worldwide this year. Notably, the US FDA granted clearances to more than 2,900 novel medical technologies in the last 12 months, highlighting a consistent stream of innovation. Among the devices witnessing highest demand are precision surgical robots, single-use disposables for infection prevention, and AI-powered diagnostic kits, each playing a role in transforming critical care pathways.
Key application areas stretch across chronic disease management, telemedicine support, and same-day surgical procedures. In cardiology, innovative ECG patches have recorded 1,200 fresh commercial launches globally in 2023, fortifying real-time monitoring in both hospital and home-based settings. Meanwhile, demand for portable ultrasound systems contributed to 78 million units sold worldwide in the past two years, primarily fueled by the need for point-of-care diagnostics in resource-limited regions. End users include large hospitals, specialized clinics, and community healthcare centers drawn to the operational efficiency and improved patient outcomes these devices offer. Consumer-facing platforms also make home-based individuals a substantial segment, accentuated by the growing adoption of smartphone-linked glucometers in diabetes care.
The global production landscape in the medical devices market is dominated by the United States, Germany, and China, with the US responsible for over 6,500 licensed device manufacturers operating in 2023. Germany exported advanced surgical instruments worth nearly US$28 billion last year, serving as a leading supplier of minimally invasive tools. China’s output of single-use medical devices, such as syringes and catheters, climbed to 65 billion units in the same period, meeting surging domestic and international demand. These countries, alongside Japan—which invests roughly US$10 billion annually in R&D for imaging instruments—shape global consumption and continually fortify their leadership in future-ready technologies.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Accelerating global reliance on wearable healthcare solutions for sustained widespread medical device interoperability worldwide
Wearable healthcare solutions in the medical devices market are redefining how patients and practitioners manage chronic conditions, track wellness indicators, and receive actionable insights. Their popularity has contributed to 504 million wearable shipments in 2023 alone, encompassing devices designed for heart rate tracking, continuous glucose monitoring, and advanced mixed-reality therapy guidance. This expansion is further evidenced by the introduction of 1,200 new smartphone-linked ECG patches this year, enabling cardiologists to diagnose arrhythmias with greater precision. US-based manufacturers account for over 40% of these cutting-edge gadgets, with nearly 2,900 fresh FDA clearances highlighting the sector’s ceaseless innovation drive. Meanwhile, India’s wearable device adoption has accelerated, reaching 75 million active users looking to improve preventive healthcare measures. Japan, known for sensor miniaturization, facilitated 600 collaborative projects focusing on unobtrusive patient-centric designs in the last two years.
Interoperability remains crucial as hospitals in the medical devices market integrate wearables into broader digital ecosystems. Developers are actively exploring software platforms that consolidate data from ECG patches, glucose monitors, and pulse oximeters into singular dashboards. In 2023, over 350 healthcare institutions in North America reportedly adopted advanced cloud-based repositories, broken down into app-friendly interfaces. Such systems allow specialists to analyze real-time vitals, cutting response times during emergencies. The transition to wearable-led monitoring not only provides continuity of care but also lowers readmission rates, which has been validated through 50 pilot programs in Europe focusing on remote discharge follow-up. Ultimately, this driver underlines a transformative movement toward less invasive, more data-driven healthcare, paving the way for broader medical device coordination.
Trend: Rapid deployment of artificial intelligence algorithms in next-generation digital diagnosis and therapeutic devices
Artificial intelligence is revolutionizing diagnostic accuracy and treatment personalization in the medical devices market. In 2023, over 200 AI-algorithm-based medical imaging software solutions received regulatory approvals across Europe and North America, improving everything from early tumor detection to orthopedic surgery planning. Radiology departments worldwide have incorporated 1,700 new AI imaging analysis systems, delivering faster detection of microcalcifications and streamlined interpretation of high-resolution scans. Robotics has likewise benefited from AI integration, as evidenced by the 7.3 million robotic-assisted surgical procedures performed worldwide in the past two years. Furthermore, advanced AI modules that interpret real-time electroencephalography have appeared in 90 neurological centers, enhancing seizure detection and expediting clinical decision-making.
Besides diagnostics, AI-powered therapeutic tools are evolving with specialized rehabilitation platforms capable of tailoring exercise protocols based on cognitive feedback. In the last 12 months, 280 clinical trials incorporating AI-based therapies have jumped from pilot to multi-center phases, reflecting significant endorsement from global health authorities. Notably, these solutions in the global medical devices market tap into big data repositories: 65 leading medical institutions pooled anonymized patient data to develop predictive models, aiming to anticipate adverse drug reactions and reduce hospitalization rates. The shift toward AI-driven medical devices aligns with the growing acceptance of machine learning in clinical workflows, as proven by 550 published studies on AI-augmented patient monitoring from January to August 2023. This burgeoning trend underscores the healthcare sector’s commitment to efficiency, accuracy, and proactive intervention—all anchored by the ongoing adoption of sophisticated, self-learning technologies.
Challenge: Addressing complex cybersecurity threats compromising connected health infrastructures amid escalating device data vulnerabilities
Securing connected health infrastructures has become a critical concern, as more than 2,000 hospital networks reported at least one major cyber threat in 2023. Medical devices in the medical devices market ranging from insulin pumps to large MRI systems are susceptible, with 600 documented intrusions specifically targeting device data transmissions this year. These sophisticated attacks bypass conventional firewalls, prompting major manufacturers to release 50 emergency software patches designed to eliminate vulnerabilities in device operating systems. Healthcare providers are accelerating the deployment of encrypted channels, with 300 leading hospitals upgrading their network protocols to mitigate interception risks during teleconsultations or remote monitoring sessions.
In tandem, regulators are pressuring manufacturers to adopt rigorous cybersecurity measures throughout a device’s lifecycle. The US FDA issued 12 new cybersecurity guidance documents covering post-launch updates, while European policymakers introduced real-time threat assessments that require device makers to report incidents within 72 hours. Bolstering readiness, nearly 140 cybersecurity audit firms now specialize in medical device risk evaluations, helping hospitals and clinics in the medical devices market spot hidden flaws in network configurations. Moreover, the rise of 5G connectivity compels device makers to account for faster data exchange that can attract more frequent intrusion attempts, and 35 industry-led consortia have formed to standardize encryption frameworks that align with next-generation network speeds. Addressing this challenge necessitates a collective endeavor, where each stakeholder—inventor, hospital, insurer, and regulator—coordinates to protect the integrity of vital medical data and safeguard patient well-being on a global scale.
Segmental Analysis
By Products
Patient monitoring devices remain the fastest-growing product category in the global medical devices market, recording a notable CAGR of 7.3% as of 2024. These devices are valued for their real-time tracking of critical vitals such as heart rate, blood pressure, oxygen saturation, and respiratory rate, which allows both clinicians and patients to respond swiftly to health changes. According to a 2023 American Hospital Association (AHA) annual report, more than 2,000 hospitals in North America have deployed wireless-enabled capnography monitors, reflecting the steady shift toward remote and continuous monitoring. Additionally, a recent survey by Emergo by UL highlights that 49% of medical device companies named regulatory compliance as a major challenge in 2022. High-acuity monitors in Intensive Care Units have also witnessed increased adoption: a 2023 Asia-Pacific Medical Device Council study showed a double-digit surge in ICU demand for advanced monitoring hardware.
This growth of the medical devices market is further bolstered by innovative launches: during the first half of 2023, over 40 new remote patient monitoring devices achieved CE Mark approval in Europe, illustrating robust acceptance of telemedicine-driven solutions. In the United States, the FDA cleared at least 30 advanced anesthetic systems by Q4 2023, emphasizing enhanced safety features and user-friendliness. Portable monitors that help reduce hospital readmission rates by nearly 18%—as estimated in a 2023 Frost & Sullivan report—have garnered particular interest from home healthcare providers. Coupled with rising usage of pulse oximeters—which, according to the International Oximetry Alliance, grew by 27% globally in 2023—manufacturers are meeting increased demand from both inpatient and outpatient settings. Overall, patient monitoring devices are positioned to remain a critical pillar of next-generation healthcare, reflecting how technology and data-driven functionalities can enhance patient welfare and reduce clinical burden.
By Applications
Among application segments, the respiratory category currently holds one of the fastest growth trajectories in the medical devices market, fueled by the rising prevalence of chronic respiratory conditions worldwide. As of 2024, devices such as nebulizers, ventilators, and Continuous Positive Airway Pressure (CPAP) machines are widely embraced in both hospital and homecare settings. A 2023 World Health Organization (WHO) update revealed that global diagnoses of chronic obstructive pulmonary disease (COPD) increased by over four million cases in just one year. In response, more than 50 newly developed ventilator models entered clinical trials globally in 2023, showcasing improvements in portability and patient comfort. Furthermore, advanced CPAP machines with integrated smart sensors—tracked by the International Sleep Foundation—have gained traction in addressing sleep apnea, which affects millions worldwide. These developments underscore how respiratory devices remain pivotal in managing respiratory distress, infection control, and long-term patient stability.
The cardiovascular segment in the medical devices market also plays a crucial role in shaping modern medical care. Pacemakers, stents, defibrillators, and other cardiac devices have consistently advanced in functionality over the past two years, integrating better battery life, remote diagnostics, and patient-friendly designs. For instance, in 2023 alone, approximately 65 novel pacemaker designs were granted approval within major regulatory markets, according to a joint statement by the European Society of Cardiology and the U.S. FDA. Meanwhile, stent technology has evolved to feature more biocompatible materials: a 2023 research publication in the Journal of Interventional Cardiology highlighted that biodegradable stent coatings significantly reduced the occurrence of post-operative complications. Furthermore, defibrillators equipped with wireless connectivity have been installed in over 1,000 U.S. community centers, reflecting broadening public safety initiatives. Taken together, respiratory and cardiovascular devices continue to drive transformative care innovations and improve life expectancy worldwide.
By End Users
Hospitals and surgical centers remain the foremost end users of medical devices market, holding around 51% of the market’s shareholding in 2024. Their dominance stems from increasing healthcare budgets, modernization projects, and a need to provide comprehensive care. The American College of Healthcare Executives noted in a 2023 analysis that nearly 200 newly built or expanded hospitals in North America have each invested in up to 15 distinct categories of high-end medical devices, ranging from robotic surgical systems to state-of-the-art imaging tools. Simultaneously, Europe’s ongoing focus on universal healthcare has spurred increased adoption of multifunctional monitoring equipment, highlighted by a 2023 MedTech Europe survey that noted a substantial rise in integrated vital signs monitors across top-tier hospitals. These trends confirm that institutional buyers continue to drive revenue and innovation within the medical devices landscape, ensuring cutting-edge solutions are readily available where patients need them most.
Other end-user segments in the medical devices market, including diagnostic centers, home healthcare settings, and ambulatory surgery centers, also fuel overall market progression by broadening access to care. For instance, home healthcare adoption of portable imaging devices has soared, with 2023 data from the Global Homecare Alliance showing nearly 10,000 newly registered home-based ultrasound units worldwide this year alone. Ambulatory surgical centers offer more affordable, outpatient-focused treatment, prompting a surge in device procurement tailored to minimally invasive procedures. As a result, at least 3,000 such centers across Asia, Europe, and the Americas have doubled their inventory of advanced endoscopic technologies since 2022 in the global medical devices market. Diagnostic centers are equally integral; in 2023, the College of American Pathologists reported that an additional 1,500 centers introduced precision-based molecular diagnostics equipment, enhancing early disease detection.
To Understand More About this Research: Request A Free Sample
Regional Analysis
Asia Pacific is set to register the highest CAGR of 7.1% in the global medical devices market, driven by strong manufacturing bases, rising adoption rates, and supportive government policies. China proves integral to this uptrend: as of 2023, it produces over 5,000 categories of medical devices, spanning everything from surgical sutures to advanced imaging equipment, based on data from the National Medical Products Administration. Japan, likewise, boasts more than 150 dedicated medical device research institutes, as per the Japan External Trade Organization’s 2023 records, each focusing on innovative solutions like robotic surgery and AI-driven diagnostics. These large-scale production capabilities foster a consistent supply of reliable products across the region.
India’s thriving medical technology landscape further cements Asia Pacific’s leadership, with over 800 indigenous start-ups dedicated to low-cost, high-impact healthcare solutions, according to the Indian Ministry of Science and Technology’s 2023 updates. Government-led initiatives in Singapore medical devices market have launched at least 40 pilot programs to embed AI-assisted health monitoring systems into public hospitals. South Korea’s medical device exports feature nearly 20,000 unique product lines, facilitated by robust R&D and technology transfer, noted in the Korea Medical Devices Industry Association’s latest survey. Additionally, Australia’s Therapeutic Goods Administration included around 2,500 new devices on its registry in 2023, many intended for outpatient and community use—indicating an expanding market aligned with evolving patient care approaches.
Throughout Asia Pacific medical devices market, collaborative efforts also steer demand: at least 12 countries signed agreements under the Asia-Pacific Economic Cooperation (APEC) forum in 2023 to simplify cross-border distribution of pivotal medical tools. Taiwan’s 25 specialized manufacturing clusters produce high-grade consumables, while Vietnam has tripled its supply of diagnostic imaging equipment since 2021, according to Vietnam’s Ministry of Health. Meanwhile, the Philippines launched a nationwide e-health monitoring framework in 2023, covering 30 million rural residents, as reported by its Department of Health. This cohesive emphasis on innovation, localized production, and accessible healthcare services positions the Asia Pacific region at the forefront of global medical device consumption, shaping a fertile landscape for sustained growth and ongoing technological breakthroughs.
Top Companies in Medical Devices Market:
- 3M Co.
- Abbott Laboratories
- Allergan Inc.
- Baxter International Inc.
- Bayer
- Becton, Dickinson and Co.
- Boston Scientific Corp.
- Cardinal Health Inc.
- Covidien plc
- Cryolife Inc.
- Danaher
- Depuy Synthes
- Endologix, Inc.
- Essilor International SA
- Fresenius Medical Care AG & Co. KGAA
- GE Healthcare
- Getinge Ab
- Johnson & Johnson
- Koninklijke Philips NV
- Medtronic Inc.
- Novartis AG
- Olympus Corp.
- Roche Diagnostics
- Siemens Healthcare
- Smith & Nephew PLC
- Smiths Medical
- St. Jude Medical Inc.
- Stryker Corp.
- Terumo Corp.
- Thermo Fisher Scientific
- Zimmer Holdings Inc.
- Other Prominent Players
Market Segmentation Overview:
By Product
- Diagnostic Devices
- Electrodiagnostic Devices
- Ultrasound Systems
- Magnetic Resonance Imaging (MRI)
- Electrocardiographs
- Scintigraphy Apparatus
- Other Electrodiagnostic Devices
- Radiation Devices
- CT Scanners
- Other Medical X-ray Apparatus
- Imaging Parts & Accessories
- Contrast Media
- X-ray Tubes
- Medical X-ray Film
- Other Imaging Parts & Accessories
- Electrodiagnostic Devices
- Consumables
- Syringes, Needles & Catheters
- Syringes (with/without needles)
- Tubular Metal Needles/Needles for Sutures
- Others
- Bandages & Dressings
- Adhesive Medical Dressings
- Non-adhesive Medical Dressing
- Suturing Materials
- Other Consumables
- Surgical Gloves
- Ostomy Products
- Blood-Grouping Reagents
- First-aid Boxes & Kits
- Syringes, Needles & Catheters
- Patient Aids
- Portable Aids
- Hearing Aids
- Pacemakers
- Therapeutic Applications
- Therapeutic Respiration Devices
- Mechano-Therapy Devices
- Others (Endoscope, Defibrillators, CRT Devices, etc.)
- Orthopedics and Prosthetics
- Fixation Devices
- Artificial Joints
- Other Artificial Body Parts
- Dental Products
- Dental Instruments and Supplies
- Dental Instruments
- Dental Cement
- Teeth and Other Fittings
- Dental Care Equipment
- Dental Drills
- Dental Chairs
- Dental X-Rays
- Dental Instruments and Supplies
- Patient Monitoring Devices
- Dental Products
- Portable Aids
- Other Medical Device Categories
- Ophthalmic Instruments
- Hospital Furniture
- Wheelchairs
- Medical & Surgical Sterilizers
- Ultra-violet or Infra-Red Ray Apparatus
- Other Instruments & Appliances
By Application
- Oncology
- Cardiology
- Orthopedics
- Ophthalmic
- Respiratory
- Urology & Gynecology
- General & Plastic Surgery
- Dental
- Diabetic Care
- Wound Management
- Nephrology
- General Hospital and Healthcare
- Ear, Nose, and Throat
- Others
By End-User
- Hospitals & Surgical Centers
- Clinics
- Household
- Other End Users
By Geography
- North America
- The U.S.
- Canada
- Mexico
- Europe
- The UK
- Germany
- France
- Italy
- Spain
- Poland
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
View Full Infographic
REPORT SCOPE
| Report Attribute | Details |
|---|---|
| Market Size Value in 2024 | US$ 517.86 Bn |
| Expected Revenue in 2033 | US$ 905.56 Bn |
| Historic Data | 2020-2023 |
| Base Year | 2024 |
| Forecast Period | 2025-2033 |
| Unit | Value (USD Bn) |
| CAGR | 6.4% |
| Segments covered | By Product, By Application, By End-User, By Region |
| Key Companies | 3M Co., Abbott Laboratories, Allergan Inc., Baxter International Inc., Bayer, Becton, Dickinson and Co., Boston Scientific Corp., Cardinal Health Inc., Covidien plc, Cryolife Inc., Danaher, Depuy Synthes, Endologix, Inc., Essilor International SA, Fresenius Medical Care AG & Co. KGAA, GE Healthcare, Getinge Ab, Johnson & Johnson, Koninklijke Philips NV, Medtronic Inc., Novartis AG, Olympus Corp., Roche Diagnostics, Siemens Healthcare, Smith & Nephew PLC, Smiths Medical, St. Jude Medical Inc., Stryker Corp., Terumo Corp., Thermo Fisher Scientific, Zimmer Holdings Inc., Other Prominent Players |
| Customization Scope | Get your customized report as per your preference. Ask for customization |
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST

 | Report ID: AA1021097 | Delivery: 2 to 4 Hours
| Report ID: AA1021097 | Delivery: 2 to 4 Hours 
.svg)






