Lateral flow assay market: By Product & Services (Readers and LFA Kits); Indications (Infectious Diseases, Pregnancy Test, and Drug of Abuse Testing); Technique (Sandwich Assays, Competitive Assays, and Multiplex Detection Assays); End User (Hospitals & Clinics, Diagnostics Laboratories, Home Care Settings, and Others); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Supermarkets/ Hypermarkets, and E-commerce); Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2024–2032
- Last Updated: 28-Nov-2024 | | Report ID: AA0522239
Market Scenario
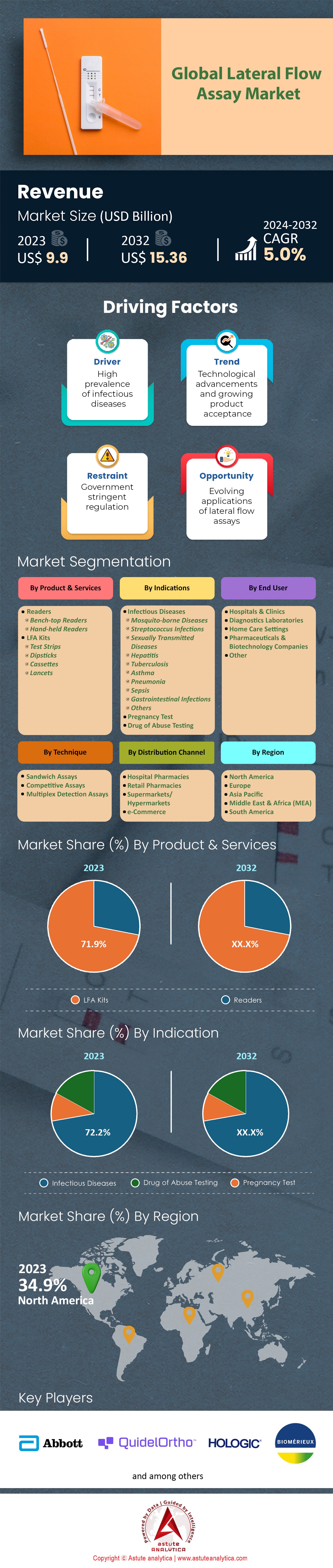
Lateral flow assay market was valued at US$ 9.9 billion in 2023 and is estimated to reach valuation of US$ 15.36 billion by 2032 at a CAGR of 5.0% over the forecast period 2024-2032.
Lateral flow assays (are low cost, rapid testing devices that make it possible to test for an analyte’s target in a sample without amassing specialized and expensive apparatuses. All tests based on this principle, where antibodies on test strips capture and label analytes providing a visible signal, are known as Immunochromatography. The importance of LFAs increased due to their unique use in point of care testing which was explicitly demonstrated during the COVID-19 pandemic where the need for quick antigen tests became crucial. For example, in 2023, over 2 billion COVID-19 antigen tests utilizing LFA technology were distributed globally, which speaks to their global use.
The lateral flow assay market are quite useful in a variety of areas including clinical diagnostics, veterinary practice, food safety and environmental science. Looking at the clinical aspect, it is evident that lateral flow assays (LFAs) are common diagnostics tools in testing for various infectious diseases such as HIV, malaria and more recently Covid-19. By 2023, about 70 million LFA tests were carried out on HIV in the world to help with advanced treatment. In a veterinary context, rapid disease tests for avian flu are one of the applications where millions of tests are done every year to protect animals. Moving onto food safety, the LFA tests also enable for pathogen and toxin detection in food to test for safety standards, they could for instance test for more than 10 million LFA based foodborne pathogens tests in the year 2023.
With regards to hospitals, LFAs are used in decision making in relation to the care of patients with hospitals and clinics being one of the key users of the LFAs. Additionally, around 500 million LFAs were used in households in 2023, given to an increase in the consumer demand for self-tests especially for pregnancy and infectious diseases tests. As For the food industry, the LFA tests have enabled more than 15000 food businesses in 2023 to comply with safety regulations. The expansion of the lateral flow assay market is fueled by factors such as the increasing incidences of infectious diseases, an expanding population of elder adults suffering with chronic diseases, and the need for inexpensive, point of care tests. The rise in demand for well-functioning diagnostic tools was proportionate to the increase of the elderly population which surpassed 750 million in the year 2023. The companies operating in this region are countering these developments through research to increase the sensitivity and specificity of LFA. For example, the American companies spent more than US$ 500 million in 2023 for the improvement of LFA methods.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Rising prevalence of infectious diseases necessitating rapid diagnostics
Increase in global cases of infectious diseases is the main factor fueling growth of lateral flow assay market. Infectious diseases continue to be among the top causes of disability and deaths, with total deaths from such diseases exceeding 15 million in 2023. The rise of pandemics such as COVID-19, tuberculosis, and HIV has increased the pressure for such tests to be developed that will be accurate and fast. For instance, there were around 10.8 million new cases of tuberculosis in 2023 where finding out the infection as early as possible is very effective in controlling the spread of the disease. On the other hand LFAs overcome the challenge of providing immediate answers at the health facility without the need for laboratory facilities which is critical in areas with scarce resources. In sub-Saharan Africa alone, vaccines facilitated over 50 million tests in 2023 which made it easier for the initiation of management of the problem. The fact that LFAs are easy to use and do not require long training makes it possible to make a great impact at the fight of the spread of infectious diseases.
With the threat of new pathogens in the lateral flow assay market also comes the need to be prepared and with diagnostic tools that don’t necessarily need to be inventive. In 2023, more than $ 1 billion worth of Orders were placed on the Lateral Flow Market and these were aimed at coming up with workable tests that target AIDS and other outbreak risk epidemic. There is rising pressure on Lateral Flow Assays from governments and health policy authorities to strengthen the future surveillance and response strategy.
Trend: Integration of digital technology with LFAs for improved result interpretation
The collaboration of lateral flow assay and the digital technologies is key trend being witnessed in the lateral flow assay market. Artificial intelligence in 2023 saw the introduction of over 150 new LFA products that can connect with smartphones and digital readers. These improvements facilitate the interpretation of results, minimize mistakes by the user, and permit data collection for epidemiological purposes. For example, using a digital LFA for COVID 19, one can now scan test strips using a mobile app which does the rest in providing an outcome while seamlessly uploading the data to health databases making it inaccessible. This digital incorporation completely shifts focus toward remote monitoring and telemedicine. In remote regions with fewer healthcare professionals available, patients are able to self-test thanks to digital LFA and a lab specialist would advise them through video call. In 2023, approximately 30 million of the digital LFA tests were employed during telehealth sessions, signifying an increasing trend of telehealth dependence.
The trend also helps in the aggregation of massive amounts of information relating to the population and therefore aiding in the surveillance of disease and their control. Such LFAs with real time information have helped in determining the influenza situation over the year 2023 which recorded over five million data most from world over. This combination of diagnostics and digital revolution is driving the lateral flow assay market towards more smart and connected healthcare systems.
Challenge: Limited sensitivity compared to laboratory-based tests affects diagnostic accuracy
LFAs are praised for the provision of quick results with minimal time being taken, however, their analytical sensitivity ranges lower than expected which can lead to false negatives in severe cases. In the year 2023, studies were conducted highlighting that some COVID-19 LFAs had a detection rate around 80 percent having a missed cases rate of around 20 percent for asymptomatic cases. Such limitations in the lateral flow assay market together raise the already present doubts on the reliability of LFAs in detecting low levels of antigens or antibodies. This issue creates a challenge for clinical decision making and control of the disease. It is especially alarming if that disease is one which can be lethal if not diagnosed at an early stage such as the case of HIV as missing out on key diagnostics can be detrimental to the battle of any public health infrastructure.
It was estimated at the end of 2023 that approximately 1.5 Million patients suffering from HIV were added thus for the future relying on such tests for their detection which is less sensitive will not prove to be efficient. In response, companies are aiming to enhance assay design and use more sensitive detection techniques. In 2023 it was estimated more than one million dollars were allocated towards research in the area of improving the sensitivity of LFAs. This includes the employing of nanoparticles and techniques which enhance the signal. It is expected from the regulatory bodies to ensure that there are stringent requirements on the LFAs which are submitted for approval adding to the challenge of the manufacturers.
Segmental Analysis
By Product
In 2023, the lateral flow assay market is largely dominated by LFA kits and their market share exceeds 71.9% because of their immediate applicability and user-friendly nature. The increasing need for rapid and easy to use point-of-care diagnostic solutions have made LFA kits to dominate the market. For example, the global sales of LFA kits for 2023 reached 2 billion units which means that they can greatly contribute towards improving healthcare services around the globe. They are simple to use and are introduced even in areas where there is no technical expertise or need for specialized equipment training. This broadens the range of users as compared to reader devices which are mostly used in laboratories under specific technical requirements.
The key reasons that LFA kits are favored over others in the lateral flow assay market include the low cost, compact design and the fast results that they provide in situations where time is critical. An average LFA kit would cost around US$ 1-5 giving people in low resource areas a chance to buy these kits, while readers start from a cost around 1000 dollars and up. As of 2023, LFA kits were used in more than 75% of rapid tests making it possible to make a diagnosis in real time which aids in infection control and disease management. In addition, LFA kits have a shelf life of two years when stored in room conditions eliminating the cost of refrigeration. Today, LFA kits are particularly used by hospitals, clinics and even the family. It’s estimated that hospitals and clinics alone utilized more than 1.5 Billion LFA kits in the year 2023 on patients for improved care outcomes. LFA kits have also been embraced by home users for self-testing, for instance, over 300 million units of home pregnancy kits were sold throughout the globe in the year 2023. With the LFA kits which provide instant results, people are able to use it to make better health choices. Health institutions and international organizations also make good use of LFA kits in mass screening exercises in areas which are experiencing epidemics.
By Indication
With a market share of over 72.2%, lateral flow assay market gains strong demand from the diagnose of infectious diseases. This is due to their speed, affordability, and ease of use. Such tests are essential for point-of-care strategies since they allow healthcare personnel to make decisions promptly without complicated laboratory designs. LFAs prove to be useful in situations with scarcity of resources and also during epidemics where there is a need to detect infections quickly. For example, LFAs became prominent in areas endemic with malaria and were able to assist in the early diagnosis of over 229 million cases of malaria reported worldwide in 2019.
Across the globe, there are still problems associated with infectious diseases. The spectrum of infectious diseases includes, but is not limited to, HIV/AIDS, Tuberculosis (TB), Malaria and COVID-19. In 2023, around 39.9 million people were reported to be living with HIV out of which 1.5 million are new infections. TB in the lateral flow assay market infected more than 10.8 million people across the globe and killed around 1.4 million. Since the outbreak of Covid-19, there have been around 180 million reported cases with nearly 4 million deaths reported as of July 2021. In this regard, LFAs were key in the rapid diagnosis of these diseases, COVID-19 antigen rapid test is one of the LFA tests that have greatly assisted in the broad-based testing of the population to help control the virus.
LFAs are advantageous to the end-users by reducing the time required for treatment of patients and the subsequent spread of infection to 15-30 minutes. They are also light and require little training, hence suitable for the use in different environments including the rural areas. Even though there are various expenditures diminutive focusing the infectious disease detection and diagnosis. The infectious disease diagnostics market penetration crossed $25B in value in the year 2020. This cost and use of LFAs assist the consumers and the health care systems cut costs as well as improving disease monitoring and control measures around the world.
By Technique
As of 2023, the sandwich assay technique is the most prominent method employed in lateral flow assay market, outpacing competitive assays and multiplex detection assays by capturing nearly 48% market share. This prominence is attributed to its high sensitivity and specificity in detecting larger molecular weight analytes, such as proteins and hormones. It is the key technology for the majority of the Enzyme Linked Immuno Assays (ELISA) test/diagnostic techniques, of which pregnancy test kits and rapid antigen tests for infectious diseases (RAETs). In 2023, over 2 billion sandwich LFA tests were done throughout the world, which showcases the penetrability of this technology in the healthcare system. It appears that the sandwich assay has never been dealt with the issues of generating false positive or negative results.
Competitive assays in the lateral flow assay market have the disadvantage of having low sensitivity to small molecules inapplicable to antigens. However, their specificity can be important when less dominant or competitive assays are used. Overlapping issues of cross reactivity and complexity in interpretation are the key limitations faced by multiplex detection assays. Its novelty means that using two antibodies to sandwich the target making the assay simple, effective and reliable with minimal experimentation required. The global pandemic combined with rapid medical advances has led to high demand for diagnostics which, as a result, has driven the demand for sandwich assays. Global investments into world sandwich assays as of 2023 have surpassed $1.2 billion suggesting that the people still have great faith in the industry. Therefore, it is expected that this technique is going to be in use for the foreseeable future with even estimates saying annual usage may exceed 2.5 units billion by the year 2025.
By End Users
In 2023, hospitals and clinics have reinforced their status as the largest end users of lateral flow assay market with a market share surpassing 38.8%. This ability can be attributed to the need for rapid diagnosis testing in these sites in order to facilitate timely clinical treatment procedures. Approximately 1.8 billion LFA tests were estimated to have been performed by hospitals and clinics across the globe within 2023. The utilization of these assays was especially employed in the detection of infectious diseases, cardiac markers and other key health indicators. The high patient volume in these institutions to touch on the need for prompt and adequate point-of-care testing some of which LFA provide as they give results within a few minutes.
Some of the reasons that explain why hospitals and clinics are using LFAs more than diagnostic labs, home care, and pharmaceutical companies include the former’s low cost, speed, simplicity, and low cost. In contrast to standard laboratory testing which may take weeks or days waiting for the results, LFA allows quick results without the use of equipment or skilled professional. The average time an LFA test is expected to take at a hospitals is about 15 minutes. In fact, the cost of an LFA test varies between $5 and $20 meaning it is cost effective for extensive testing. Also, more than 70% [approximately 676 hospitals] of hospitals have incorporated LFAs in their emergency in order to improve the Triage of patients at the ER’s.
Worldwide, the explosive growth of infectious diseases as well as the focus on early diagnosis put hospitals and clinics in the forefront of the use of the lateral flow assay market. As of 2023, countries around the world spend over US$ 70 billion on infectious disease diagnostics. On a microeconomic level, the use of LFAs, in the case of hospitals and clinics, decreased the patient’s waiting time by up to 30%, thus increasing the level of contentment in patients and improving the operational efficiency of these institutions.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
To Understand More About this Research: Request A Free Sample
Regional Analysis
Lateral flow assay is now on the upward trend since many regions across the world have transformed this market. These regions include North America, Europe and Asia Pacific. North America is ranked first in this market by accounting for more than 34.9%, which is followed by Asia Pacific. Wherein, increased investment in healthcare infrastructure, rapid spread of COVID-19, and a growing proportion of the population suffering from chronic diseases in the US are the foremost reasons that extend lateral flow assay market dominance in the region. It has an advanced healthcare system, large amounts of capital funding deployed into the marketing of the United States healthcare system. In 2023, the total amount of lateral flow tests conducted in the US alone surpass a whopping 1.2 billion, encompassing targets even diabetes as well as mega health crises such as the COVID pandemic and Influenza as well. Another pivotal factor is Canada where tests exceeded more than 1.2 billion over the past year alone as the government was able to finance more than 200 LFA related diagnostic innovations aimed at rural areas in 2023.
The lateral flow assay market in Europe is quite promising as the region shows a strong commitment to the adoption of integrated early diagnosis and treatment strategies. Countries spearheading this trend include Germany, United Kingdom, and France with Germany in 2023 alone using more than 25 million lateral flow tests across clinical and domestic settings. At the same time, the efforts of the European Commission in promoting convergence of rules across the Union have also facilitated the uptake of LFAs especially for infectious diseases and pregnancy testing. Moreover, the region’s population structure has been a driver for the need of quick - acting and painless diagnostic instruments, more than 40% of the health centers in Europe, today, incorporate lateral flow assays in standard diagnostics work. The United Kingdom has become an epicenter for LFA development, at least 50 spin off companies have been created in 2023 focused on the development of the next generation of assays for oncological and cardiovascular diseases.
Asia Pacific is the fastest growing region in terms of revenue and demand for lateral flow assay market, owing to increasing government initiatives, infectious diseases burden, and growing awareness about health care. In 2023 a combined of India and China conducted millions of lateral flow tests as a result of government campaigns on tuberculosis and COVID -19 tests.
Top Companies in Lateral Flow Assay Market:
- Abbott Laboratories
- bioMerieux S.A
- Bio-Rad Laboratories Inc.
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- Hologic Inc.
- Merck KGaA
- PerkinElmer Inc.
- Qiagen N.V.
- Quidel Corporation
- Siemens Healthineers
- Thermo Fisher Scientific, Inc.,
- Other Prominent Players
Market Segmentation Overview:
By Product & Services:
- Readers
- Bench-top Readers
- Hand-held Readers
- LFA Kits
- Test Strips
- Dipsticks
- Cassettes
- Lancets
By Indications:
- Infectious Diseases
- Mosquito-borne Diseases
- Streptococcus Infections
- Sexually Transmitted Diseases
- Hepatitis
- Tuberculosis
- Asthma
- Pneumonia
- Sepsis
- Gastrointestinal Infections
- Others
- Pregnancy Test
- Drug of Abuse Testing
By Technique:
- Sandwich Assays
- Competitive Assays
- Multiplex Detection Assays
By End User:
- Hospitals & Clinics
- Diagnostics Laboratories
- Home Care Settings
- Pharmaceuticals & Biotechnology Companies
- Other
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Supermarkets/ Hypermarkets
- e-Commerce
By Region:
- North America
- The U.S.
- Canada
- Mexico
- Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)