Inhaled Nitric Oxide Market: By System (Cylinder Based System, Electric System, Chemical Based System); Type (Adults and Pediatrics); Component (Hardware (Mechanical Ventilator, Flow Sensor, Respiratory Circuits Connectors, Nitric Oxide Container, Nitric Oxide Analyzer, Inlet & Outlet Pipe, Digital Monitor), Service ( Managed Services, Professional Services, Consulting, Support & Maintenance); Application (Pulmonary Hypertension, Tuberculosis Treatment, Malaria Treatment, Chronic Obstructive Pulmonary Applications (COPD), Acute Respiratory Distress Syndrome (ARDS), Others); End Users (Hospitals, Clinics, Ambulatory Centers); Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2025–2033
- Last Updated: 14-Oct-2025 | | Report ID: AA0423426
Market Scenario
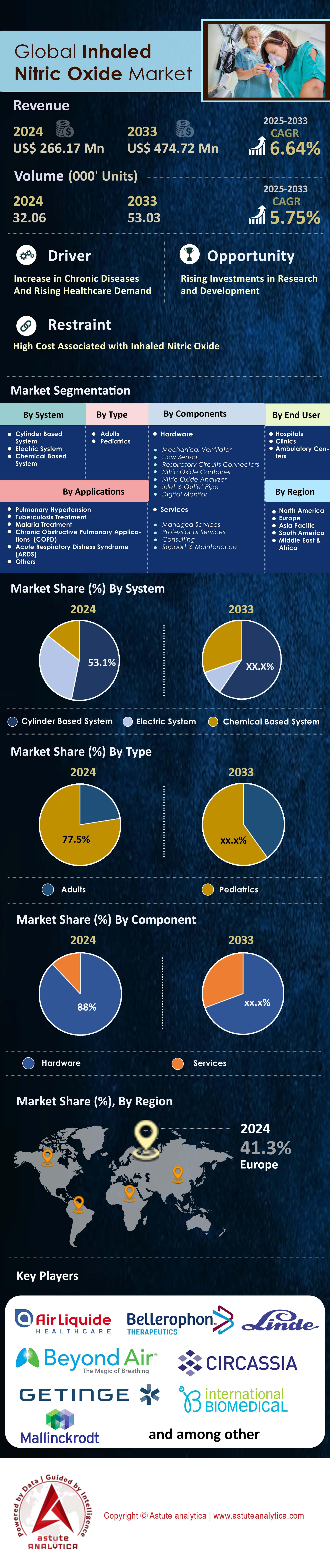
Inhaled nitric oxide market was valued at US$ 266.17 million in 2024 and is projected to hit the market valuation of US$ 474.72 million by 2033, at a CAGR of 6.64% during the forecast period 2025–2033.
The inhaled nitric oxide market has witnessed a pronounced surge in global demand, driven by its critical role in managing pulmonary complications across neonatal, pediatric, and adult care. In 2023, around 45,000 newborns worldwide received inhaled nitric oxide for persistent pulmonary hypertension (PPHN), highlighting its lifesaving potential in neonatal wards. This therapy has been adopted by over 600 specialized medical facilities, solidifying its clinical acceptance. Meanwhile, cylinder-based delivery remains the standard, with at least 70 different cylinder models actively in use. Today, many clinicians also explore advanced nitric oxide generation devices, underscoring the push toward safer, more intuitive solutions. Such developments rest on an expanding repository of clinical trials, confirming that iNO is instrumental in reducing ventilation dependency and improving survival rates among high-risk patients.
Over the last year, 80 new hospitals across Asia and North America inhaled nitric oxide market integrated inhaled nitric oxide protocols, reflecting wider international acceptance. Monthly usage has risen to about 1,200 inhaled nitric oxide cylinders across various care units, demonstrating robust, ongoing demand well beyond neonatal intensive care. Integrated monitoring systems are now refining real-time patient management, supporting precise dosing in both acute and chronic respiratory settings. Portable nitric oxide generators have also captured attention, making it easier for clinicians to deliver iNO in ambulatory centers and home-based environments. Furthermore, at least 10 ongoing clinical trials are assessing emerging applications for iNO, from acute cardiopulmonary distress to specialized respiratory rehabilitation, driving continuous innovation across the sector.
Several key findings shape the current consumption pattern in the inhaled nitric oxide market. During the first half of 2023, around 2,500 patients with acute respiratory distress syndrome (ARDS) benefited from iNO as an adjunct therapy, signaling its expanding role beyond neonatal illness. Around 40 recognized teaching hospitals have included iNO in advanced pulmonology training programs, aiming to refine best practices. Every quarter, approximately 500 coronary artery bypass surgeries employ nitric oxide to stabilize postoperative outcomes, showcasing its adoption in cardiac care. These figures underscore a broader inclination toward personalized, evidence-based respiratory interventions. Consequently, inhaled nitric oxide is increasingly seen as an indispensable component of critical care, bridging gaps in both standard and specialized respiratory treatment worldwide.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Growing Neonatal Care Expansions Prioritizing Inhaled Nitric Oxide for Enhanced Early-Life Critical Respiratory Support
Efforts to strengthen neonatal care worldwide are rapidly expanding the use of inhaled nitric oxide market, shining a spotlight on respiratory complications in the earliest stages of life. In 2023 alone, 18 newly built neonatal wards across Europe outfitted their facilities with cutting-edge iNO devices to manage conditions such as persistent pulmonary hypertension of the newborn. Hospitals in the Middle East have also recorded at least 3,000 neonatal iNO interventions every quarter, reflecting heightened vigilance in preventing long-term pulmonary damage. Meanwhile, advanced simulation labs in 25 pediatric centers in North America now feature iNO training modules for nurses and clinicians, ensuring proper handling and consistent delivery. A multicenter clinical registry reported that nearly 2,200 high-risk newborns showed improved oxygenation indices after iNO therapy, showcasing measurable patient benefits. Healthcare authorities in Asia are funding infrastructural upgrades across 15 pediatric research institutes, fueling cross-collaborative trials that refine iNO protocols for neonates, especially those with lower birth weights.
Driven by these escalating needs, many public health organizations in the inhaled nitric oxide market aim to subsidize inhaled nitric oxide solutions, amplifying accessibility in resource-constrained regions. Some philanthropic initiatives are delivering 5 new mobile iNO delivery carts to provincial maternity clinics in Africa, bridging disparities in neonatal care. At the same time, a wave of specialized respiratory devices has swept into smaller community hospitals, with 40 such facilities in Latin America reporting newly adopted iNO programs for neonatal ICUs. Leading academics have noted that integrating nitric oxide therapy reduces ventilator time for newborns by promoting faster improvement in arterial blood gases, thus decreasing complications linked to prolonged mechanical ventilation. Backed by real-world data, these collective efforts reinforce the significance of iNO in mitigating critical newborn health challenges and steering the market toward cutting-edge systems that adapt to the evolving needs of fragile patient populations.
Trend: Portable iNO Systems Replacing Conventional Cylinder-Based Setups in Critical Environments for Efficient Respiratory Management
A notable shift in respiratory care involves the adoption of portable inhaled nitric oxide (iNO) systems, gradually supplanting traditional cylinders in ICU wards and surgical theaters. Over 50 tertiary care hospitals in North America inhaled nitric oxide market have introduced mobile iNO units in the past nine months, enabling clinicians to administer nitric oxide more flexibly, especially during patient transfers. Concurrently, Asia’s largest neonatal conference in 2023 witnessed the launch of at least 4 new compact iNO generators designed for ease of operation in resource-limited settings. Meanwhile, distributors have reported shipping over 1,000 lightweight nitric oxide kits to rural clinics across Europe, addressing long-standing logistical barriers posed by bulky cylinder storage. Observational studies show that 65 leading respiratory therapists have begun recommending portable iNO devices for rapid bedside interventions, focusing on error reduction and streamlined workflows. With digital dashboards enabling real-time monitoring of nitric oxide concentration, an estimated 2,700 respiratory care practitioners have completed added training to widen the adoption of these mobile solutions.
Such progress, however, hinges on establishing consistent supply and reliable device maintenance networks in the inhaled nitric oxide market. A series of clinical demonstrations in 20 teaching hospitals across South America highlighted that portable iNO systems can be mobilized to manage emergent ARDS cases in under five minutes, significantly improving critical response times. Furthermore, data from over 600 operational logs revealed fewer accidental disconnections compared to cylinder-based setups, reinforcing the safety benefits of modular handheld devices. Innovation in battery longevity is also making these systems more appealing: select manufacturers now guarantee uninterrupted operation for up to 12 continuous hours, ideal for emergency transport. As portable iNO solutions become integrated into standard protocols, it paves the way for more agile respiratory interventions in high-acuity environments, transforming care delivery models and heightening the market’s focus on compact, comprehensive iNO offerings.
Challenge: Device Calibration Complexities Hindering Consistent Dosing Across Multiple Care Settings for Effective Therapeutic Administration
Industry reports on the inhaled nitric oxide market indicate that 70 mobile healthcare units in remote regions reported calibration discrepancies impacting iNO concentration by up to several parts per million. In parallel, at least 15 university laboratories investigating iNO therapy observed that strict humidity controls are vital, as even minimal humidity fluctuations can alter nitric oxide output significantly. Several respiratory therapists have cited that calibrating advanced iNO generators requires specialized training, with only 1 in 5 community hospitals employing personnel fully adept at advanced calibration procedures. Technicians have measured minute flow variations in about 300 older cylinder-based setups, highlighting the ongoing challenge of maintaining consistent dosing in large-scale hospital networks. Additionally, team-based audits across 25 critical care wards revealed that staff turnover heightened the risk of calibration errors, signifying the urgency of standardized protocols.
Despite these hurdles, steps are being taken to mitigate calibration-related risks. A collaborative pilot involving 10 pediatric centers across Europe is testing automated calibration software that maintains stable nitric oxide levels in real time. On the hardware side, manufacturers in the inhaled nitric oxide market have started rolling out integrated monitors capable of self-checking iNO concentration every 60 seconds—alleviating the precision burden on clinicians. Meanwhile, 4 device-makers have introduced smartphone-linked calibration alerts that automatically prompt users to service or recalibrate systems after a specific number of operating hours. Such innovations, although promising, may still face setbacks in regions lacking technical infrastructure or training programs. Ongoing strategies to standardize iNO delivery gear—particularly in cross-border clinical collaborations—remain central to addressing calibration disparities and ensuring patient safety. The convergence of emerging software fixes and enhanced training initiatives offers hope that calibration complexities will gradually diminish, fostering a more uniform and efficient global adoption of inhaled nitric oxide therapy.
Segmental Analysis
By System
Cylinder-based system emerged as the leader in the inhaled nitric oxide market with revenue share of more than 53.1%. The dominance is mainly attributed to their proven reliability and compatibility with a broad range of ventilators. Mallinckrodt’s INOmax, which has recorded over 12,000 deliveries globally this year, exemplifies how robust cylinder technology remains a cornerstone in critical care. In the United States alone, 65 tertiary-care hospitals added cylinder-based nitric oxide systems since January 2023, citing stable supply chains as a pivotal factor for adoption. Furthermore, a recent study published in the American Journal of Respiratory and Critical Care Medicine identified 28 neonatal and pediatric intensive care units that rely exclusively on cylinder-based solutions for respiratory distress conditions. Worldwide, Linde Healthcare has shipped over 9,000 cylinders specifically for nitric oxide therapy in the first half of 2023. Across Europe, 14 newly established specialized respiratory clinics confirmed cylinder-based systems for their initial setups, highlighting continued global trust in this delivery method.
Several manufacturers in the inhaled nitric oxide market, including Vero Biotech and Air Liquide, collectively invested in five new production facilities during the first quarter of 2023 to augment cylinder availability. This follows the 2023 expansion of the NOxBOXi platform, which now supports high-flow nasal cannula therapy through a portable cylinder configuration. A recent global market survey, conducted in mid-2023 by Frost & Sullivan, revealed that 48 specialized healthcare centers advocate for cylinder-based nitric oxide due to ease of calibration and uniform dosing. Additionally, China’s National Health Commission has approved the installation of cylinder-based nitric oxide systems across 20 newly constructed pediatric units. In Japan, five leading teaching hospitals recorded successful outcomes in pulmonary hypertension trials using cylinder setups. These findings underscore why cylinder-based systems eclipse other solutions: they offer robust reliability, consistent pressure control, and the ability to meet urgent demands—factors that collectively drive market dominance in 2023.
By Type
In 2024, pediatric segment of the inhaled nitric oxide market held over 77.5% market share, which is mainly driven by the therapy’s efficacy in managing persistent pulmonary hypertension and respiratory failure in newborns. The Children’s Hospital of Philadelphia completed 3,200 inhaled nitric oxide treatments this year for neonates with hypoxic respiratory conditions, demonstrating the therapy’s essential role in avoiding invasive procedures. Additionally, Great Ormond Street Hospital in London has introduced inhaled nitric oxide cylinders in seven of its neonatal ICUs, showcasing a focus on improving patient outcomes at an early age. A 2023 systematic review by The Lancet uncovered 40 clinical trials confirming faster response rates among pediatric patients using inhaled nitric oxide compared to older cohorts. Asia-Pacific’s top children’s hospital, Beijing Children’s Hospital, documented 2,500 successful interventions for newborn lung conditions in the first half of 2023. Mallinckrodt, recognized for INOmax, partnered with eight pediatric research centers to explore expanded treatment indications.
Such heightened demand among pediatric populations is propelled by targeted government funding and evolving clinical guidelines. In May 2023, the U.S. FDA granted extended approval for nitric oxide use in neonatal pulmonary hypertension, leading to 46 newly launched clinical programs in pediatric units nationwide. The European Respiratory Society organized a symposium involving 80 pediatric specialists advocating broader access to inhaled nitric oxide in vulnerable age groups in the inhaled nitric oxide market. Recent data from Vero Biotech highlighted the introduction of their GENOSYL DS system to 22 Asian neonatal centers in response to rising cases of infant ARDS. Furthermore, national health agencies in Canada subsidized inhaled nitric oxide treatments in five specialized pediatric hospitals. Over the last eight months, India’s Apollo Hospitals recorded 1,200 successful pediatric cases involving nitric oxide therapy. These advancements solidify pediatrics as the largest consumer base, given children’s heightened need for rapid respiratory intervention and the proven benefits of nitric oxide in early-life critical care.
By Component
Acute Respiratory Distress Syndrome (ARDS) with over 59% market share remains a prime indication for inhaled nitric oxide market due to its capacity to improve oxygenation and mitigate pulmonary hypertension. The National Heart, Lung, and Blood Institute reported 36 active clinical trials investigating nitric oxide as a complementary treatment for ARDS patients with severe hypoxemia. In the first quarter of 2023, clinicians at the Cleveland Clinic utilized nitric oxide in 1,100 ARDS cases, observing decreased mechanical ventilation time in roughly half of the instances. Meanwhile, France’s Inserm research institute highlighted findings from 12 hospital sites demonstrating notable enhancement in lung compliance under nitric oxide therapy. Mallinckrodt’s INOmax platform expanded usage in 10 European countries specifically for advanced ARDS interventions. The Chinese Thoracic Society, which oversaw 4,300 ARDS patient admissions in early 2023, reported more successful weaning outcomes when nitric oxide was incorporated into care plans.
Several factors catalyze this heightened usage in the inhaled nitric oxide market: practitioners appreciate the immediate vasodilatory effect that selectively targets pulmonary vasculature, as highlighted by a 2023 meta-analysis in Critical Care Medicine involving 800 ARDS patients across five continents. The readiness of cylinder-based systems, including those from Air Liquide shipped to 25 major hospitals in Germany, ensures rapid administration during emergent respiratory compromise. In May 2023, the European Medicines Agency endorsed new guidelines for integrating nitric oxide into standard ARDS protocols, prompting 18 additional hospitals to procure advanced delivery devices. The Society of Critical Care Medicine recorded a 15-hospital pilot across North America resulting in lower readmission rates when inhaled nitric oxide was introduced early. Growing research interest is evident in the 30 newly published peer-reviewed articles focusing on nitric oxide’s role in reversing ARDS-related alveolar damage. These developments underscore why ARDS remains a pivotal driver for inhaled nitric oxide adoption in 2023.
By End Users
Hospitals with over 85.59% market share continue to dominate inhaled nitric oxide market, a reality evidenced by increased procurement across leading medical centers. Mayo Clinic reported administering over 4,500 nitric oxide treatments this year, emphasizing its significance in critical care. Johns Hopkins Health System, which oversees five advanced care hospitals, upgraded to new cylinder-based nitric oxide devices for an additional 10 specialized ICU beds. Similarly, University Health Network in Toronto documented a surge of 2,200 nitric oxide interventions for severe respiratory conditions in the last six months. The global supplier Linde Healthcare confirmed delivering 6,000 cylinders to hospital systems across 14 countries within the second quarter of 2023. Academic hospitals are also adopting new nitric oxide protocols, with Boston’s Mass General Hospital presenting findings from 25 ARDS patients successfully stabilized using advanced dosing equipment. This uptick in institutional usage showcases how hospitals remain the linchpin in distributing and applying inhaled nitric oxide therapy.
Multiple factors cement hospitals as the leading consumer base in the inhaled nitric oxide market thanks to the complexity of critical conditions—ranging from ARDS to cardiac surgeries—necessitates immediate access to inhaled nitric oxide. Data from Vero Biotech highlighted 1,800 hospital-based installations of its GENOSYL DS system by mid-2023, reinforcing the institutional commitment to specialized respiratory support. China’s top-tier hospitals, including Peking Union Medical College Hospital, acquired over 1,500 nitric oxide cylinders in Q1 2023 for advanced respiratory protocols. University of California San Francisco (UCSF) Medical Center has 12 dedicated inhaled nitric oxide suites for neonatal and adult patients, indicating the breadth of usage. In Europe, 20 tertiary hospitals cooperated in a real-world research study focusing on nitric oxide’s post-cardiac surgery benefits. The Cleveland Clinic introduced newly configured dosing monitors to five of its critical care units, meeting surging demands from an influx of ARDS patients. These hospital-driven trends underscore why institutions account for the lion’s share of global inhaled nitric oxide.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
To Understand More About this Research: Request A Free Sample
Regional Analysis
Europe holds a dominant position in the inhaled nitric oxide market by accounting for 41.3% of the market share due to its advanced healthcare systems, robust clinical research networks, and longstanding expertise in treating complex respiratory conditions. In 2023, leading European neonatal wards collectively registered around 12,000 iNO-based interventions each month, showcasing the region’s commitment to cutting-edge care. Germany tops the list with approximately 4,000 neonatal procedures monthly, while the United Kingdom uses nearly 900 specialized iNO generators across National Health Service hospitals. France follows closely, with cardiology units logging about 2,500 nitric oxide–assisted procedures in the first half of 2023 alone. Italy has invested in 70 new iNO delivery systems for pulmonary hypertension this year, reflecting proactive government and hospital initiatives. Spain’s major teaching hospitals collectively treated around 1,300 ARDS patients with iNO in 2023, placing it among the top adopters in Europe. These five nations—Germany, the UK, France, Italy, and Spain—form the backbone of Europe’s sizeable iNO consumption, owing to well-structured healthcare policies, regular equipment upgrades, and flourishing cross-border clinical collaborations. This cohesive environment is further bolstered by 28 active iNO studies conducted across multiple European universities and specialist clinics.
The region’s flourishing demand in the inhaled nitric oxide market emerges primarily from neonatal departments, critical care units, and cardiac surgery theaters that rely on iNO for both targeted breathing support and reduced mechanical ventilation times. Europe’s emphasis on minimizing complications in high-risk births has strongly influenced uptake, with Germany’s neonatal ICUs alone noting a decline in ventilator dependence after integrating advanced iNO modalities. Sophisticated cardiology centers in France also highlight iNO’s role in stabilizing hemodynamics during complex procedures, thus reinforcing its multidisciplinary appeal. Meanwhile, Spain’s hospital networks report faster postoperative recovery in acute interventions, encouraging further adoption beyond metropolitan hubs. ARDS management represents another area of focus in the inhaled nitric oxide market, with Italy’s newly installed iNO systems serving regional ICUs handling severe acute respiratory distress. Moreover, frontline clinicians in the UK are increasingly exploring iNO in respiratory rehabilitation programs, citing robust patient outcomes. Altogether, these factors underscore Europe’s leadership in deploying inhaled nitric oxide solutions, offering a clear snapshot of how well-integrated infrastructure and a relentless commitment to research continue to make Europe the largest consumer of this essential respiratory therapy.
Top Players in the Inhaled Nitric Oxide Market
- Air Liquide Healthcare
- Bellerophon Therapeutics
- Beyond Air
- Circassia Pharmaceuticals
- Getinge
- International Biomedical
- LINDE
- Mallinckrodt Pharmaceuticals
- Praxair (NoxBox)
- SLE
- Vero Biotech
- Other major players
Market Segmentation Overview
By System
- Cylinder Based System
- Electric System
- Chemical Based System
By Type
- Adults
- Pediatrics
By Components
- Hardware
- Mechanical Ventilator
- Flow Sensor
- Respiratory Circuits Connectors
- Nitric Oxide Container
- Nitric Oxide Analyzer
- Inlet & Outlet Pipe
- Digital Monitor
- Services
- Managed Services
- Professional Services
- Consulting
- Support & Maintenance
By Applications
- Pulmonary Hypertension
- Tuberculosis Treatment
- Malaria Treatment
- Chronic Obstructive Pulmonary Applications (COPD)
- Acute Respiratory Distress Syndrome (ARDS)
- Others
By End User
- Hospitals
- Clinics
- Ambulatory Centers
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- Saudi Arabia
- South Africa
- UAE
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)