Genomic Testing Market: By Offering (System and Software, Reagents & Consumables, Services); Testing Type (Sequencing Solution, Others); Technology (Proteomics, Pharmacogenomics, Stem Cell Therapy, Cloning); Indication (Cancer, Asthma, Diabetes, Hearth Diseases, Agricultural Production, Others); End-User (Hospitals & Clinics, Research Centers & Academic Institutions, Pharmaceutical & Biotechnology Companies, Others); Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2024–2032
- Last Updated: 21-Nov-2024 | | Report ID: AA0423400
Market Scenario
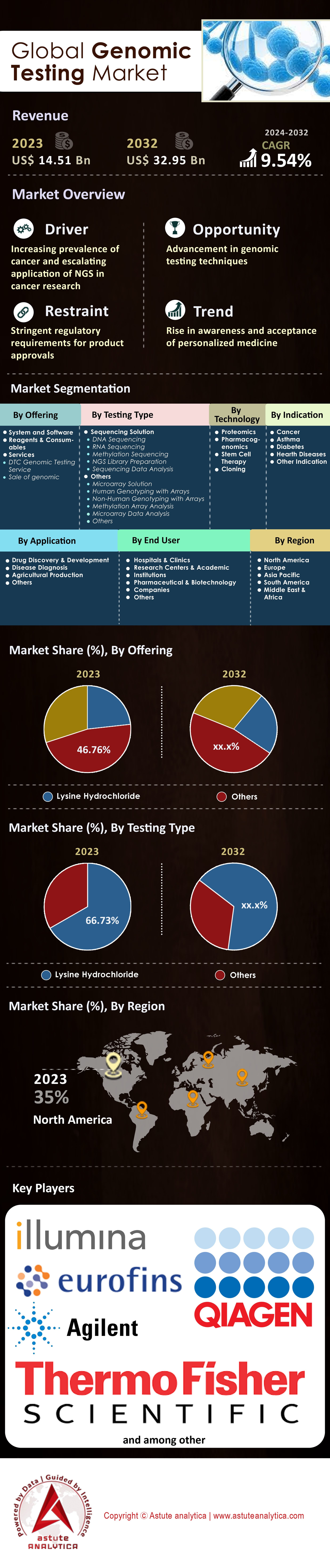
Genomic testing market was valued at US$ 14.51 billion in 2023 and is projected to attain a valuation of US$ 32.95 billion by 2032, at a CAGR of 9.54% during the forecast period, 2024-2032.
Genomic techniques have grown rapidly in the past few years, especially from 2018 to 2022. The rapid medical advancements in the market can be attributed to the increased use of genomic technology. For example, the cost of sequencing a human genome in 2023 is approximately $600 as opposed to $1000 just a few years ago. This enables more patients and healthcare providers to affordably approach genomic testing. The use of detectors promotes further progress in the deciphering of genomes by allowing thousands of samples to be processed rapidly and cheapening the amount of time needed to wait for the results. Technologies like nanopore sequencing have also increased the portability and accessibility of genetic testing, thereby increasing its uses in diverse scenarios.
Increasing prevalence of genetic diseases is one of the key factors behind the rapid growth in the genomic testing market. There are over 400 million people across the world who suffer from rare genetic disorders which means that they require advanced diagnostic techniques. Genomic testing has become vital in oncology such that in USA alone, there were over 2 million new cancer cases reported. Wherein, most of the treatments are guided by genomic profiling. Highlighting an expanding arsenal of precision medicine, FDA approved more than 50 new genomic biomarkers for cancer treatment in 2023. Moreover, genetic variability such as various strains of viruses causing infectious diseases have emphasized the significance of genomic surveillance and testing.
Furthermore, genomic testing market is driven by the rise in demand for personalized medicine that provides individualized healthcare grounded on a person’s genetic information. The genome testing market is founded on personalized medicine. In 2023 alone, pharmaceutical companies put more than $15 billion into genomic research and development to come up with tailor-made medicines. Over 5,000 clinical trials involving genomic biomarkers were carried out in 2023, showing an active pipeline of new personalized therapies under investigation. Government initiatives such as the European 1+ Million Genomes initiative, which aims to sequence over one million genomes by 2023 are indicative of global commitment toward using genomics for better health outcomes. Taken together these factors drive substantial and sustained growth in the genomic testing market establishing it as a vital element of contemporary healthcare system.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Increasing Demand for Personalized Medicine Fueling Genomic Testing Advancements Globally
The increasing need for bio marking is one of the major factors which is the reason for escalation in the global genomic testing market. Bio marking, also known as personalized medicine is tailoring the medical treatment to the individual characteristics of each patient for example, their genetic makeup. So far, more than 1 million complete human genomes have been sequenced around the globe, which contributed in sequencing targeted drugs and other methods of diagnosis. Considering the state of the world today with such as cancer or genetic disorders. In 2020, there were close to 19.3 million cancer diagnostic cases around the world demonstrating the need for effective diagnostics.
Looking at it now it is evident as to how far along genomic testing has come today, as it allowed the treatment of multiple cancer patients with the help of targeted therapies. The FDA has more than 90 targeted cancer treatments that are appropriate to be used which have gone through genetic testing and received legal approval. Another interesting statistic shows that the FDA has greater than 250 pharmacogenomic markers which further emphasizes the importance genetics holds in the future when it comes to drug response and making customized treatment plans. This transition in the genomic testing market is in addition to government plans and additional financial resources. The National Institutes of Health (NIH) in the United States in 2020 spent about $3.7 billion on genetic and genomic studies and research. Other initiatives such as the All of Us Research Program by National Institutes of Health (nih) also aim to help collect genetic information for more than a million individuals that will help in deepening the research into personalized medicine. In UK, the 100,000 Genomes Project was able to sequence a total of 100,000 whole genomes which allowed for better diagnosis of patients suffering from rare diseases.
Trend: Adoption of Next-Generation Sequencing Technologies Expanding Genomic Testing Capabilities Extensively
The implementation of Next-Generation Sequencing (NGS) technologies is significantly transforming and modernizing the scope of genomic testing market. NGS technology has made genome and exome sequencing easier and made sequencing of longer DNA strands more efficient. As of 2023, the NGS market was estimated at US$ 13.2 billion and it is poised to reach $27 billion by 2027. The market as well as its penetration in clinical practice has been bullish. The distinctive feature of NGS that permits running a massive number of DNA fragments through a sequencer in a single experiment has cut back sequencing time to a few days.
Most importantly NGS technologies in the genomic testing market have impacted a number of industries, primarily the domain of oncology and rare genetic disorders. Currently, there are more than 50 NGS-based gene panels that have been cleared for cancer diagnostics that assist in the detection of targetable mutations for therapies against cancer. With rare diseases affecting approximately 300 million persons globally, NGS allows the discovery of the genetic variants responsible for the disease and ensures patients are diagnosed effectively where other techniques have failed. Moreover, during the pandemics of COVID-19, NGS provided useful assistance, with more than 2 million SARS-CoV-2 genomes being analyzed and sequenced throughout the world by mid-2021 for tracking virus changes.
There are factors like investment and partnership in the genomic testing market that are driving the adoption of NGS to next level. The value of this technology was highlighted when NIH allocated more than $1 billion on NGS related projects in 2020. Robust market growth is also reflected in Illumina's reported revenues of $3.2 billion for the year. The clinical use of the platform such as the Illumina MiSeqDx that was approved by FDA are even easier to obtain. In addition, there are now more than 100 universities worldwide that offer programs related to genomics and NGS, providing qualified specialists needed for the development of this industry.
Challenge: High Costs of Genomic Testing Limiting Accessibility for Broader Patient Populations Worldwide
The global adoption of genomic testing market suffers significantly from the high native costs that should be controlled. Normal sequencing of the human genome went down from US$100 million in 2001 to about US$600 today. However, the expenses of the genomic resolving high amount especially when borne with the genomic interpretation costs and clinical data consultation. Whole exome targeted therapy isn’t affordable as the cost range lies between US$1000 to US$5000 which Is beyond reach for the many patients especially where there is no adequate insurance.
Attaining services for advanced genomic diagnosis in the low- and middle-income countries Is still quite a challenge. Wherein, annual spent per person on healthcare in the genomic testing market is between US$100 and US$200 making it difficult to afford services targeting genomics for the vast majority. This is true especially in middle- and high-income countries. For example, close to 28 million people in the US were uninsured in 2023. Reducing their access to such advanced diagnostics. Additionally, the costs of maintaining sequencing platforms and employing specialized personnel add to the overall expense, often limiting genomic testing services to well-funded institutions.
However, efforts to mitigate these costs in the genomic testing market are in full swing, albeit with a slew of challenges. It is encouraging to see that government programs and non-market institutions are putting in a lot of work to promote greater access to testing, but resources fall way short of the demand. There is wide variability in the reimbursement policies of insurance, and most do not reimburse genomic tests of wider scope because of costs or lack of clinical usefulness. Furthermore, costs associated with the storage and maintenance of vast genomic data sets also contribute to the cost burden. As such, without a radical decrease in the relevant costs or fresh investment, the price of genomic testing will remain an issue to the extension to wider patient groups.
Segmental Analysis
By Type
Over 46.7% of the market share is held by the reagents and consumables segment which remains a critical constituent of the genomic testing market. This segment is driven by the growing usage of genomic testing in clinical and research practice globally. In the United States alone, more than 2,000 genomic testing labs rely heavily on high-quality consumables for accurate DNA sequencing and analysis. A rise in number of automated genomic systems has increased the requirement for such consumables with more than 5000 laboratories working with such automated platforms worldwide. A huge part of this demand is catered from the North America region which sells more than 600 million consumables units every year. Furthermore, the supply of reagents specific to high throughput sequencing has also shown tremendous increase, where about 1.5 billion units are produced per year throughout the globe to support these advanced technologies.
Apart from this, Asia Pacific region has emerged a strong genomic testing market as the region imported more than 300 million units of reagents and consumables. Considering the statistics at hand, it is clear that the Asia Pacific region has surpassed others in terms of the import demand of gene sequencing in the past decade. It is suggested that this trend is bound to continue as Pacific countries, particularly those in Southeast Asia, will remain major players and consumers in the market. Units of consumables distributed across genomic research institutions in Europe have similarly soared to 450 million. In Europe, it was observed that the introduction of over 450 million consumable units in institutions doing genomic research, indicating the increased demand on the part of these institutions. With over 100 of them released every year, this shows that there is constant change in the innovations and trends that people are in need of.
By Testing Type
Sequencing solutions, more predominantly Next Generation Sequencing (NGS) technologies, remain the huge players in the genomic testing market accounting for a market share exceeding 66.73%. Within the past year, an impressive over 6 million NGS tests were carried out around the world thus reinforcing the importance of these technologies in the advancement of diagnostics. Recently, one of the biggest advances has been the development that has driven the costs of sequencing a human’s genome at $600. Thus, opening up many avenues, even opportunities for new uses. For example, oncology has been revolutionized by NGS technologies enabling over 2 million cancer patients to receive genomic profiling to adjust their treatments accordingly.
There are more than 500 NGS platforms actively in use across the world deployed for purposes such as rare disease diagnosis and drug hunting. Trust in the growing capabilities of these technologies has prompted the endorsement of more than 200 NGS-based diagnostic tests by regulatory agencies. More than 3000 NGS platforms have been adopted by universities and research institutions across the globe enabling them to undertake large genomics projects. Portable NGS devices are selling 200,000 units per year, making genomic testing possible in hard-to-reach and economically challenged populations. Furthermore, more than 1000 NGS based clinical trials are conducted all over the world as NGS has become far reaching in terms of investigations and possible treatment solutions.
By Application
The genomic testing market is expected to grow substantially in the upcoming years owing to its application in drug development and drug discovery, which accounted for more than 46% of the global revenue share. It allows researchers to get a more in-depth understanding of genetic differences that result in altered drug response and progression of disease. Over 5000 drug development projects have utilized genomic data to improve their odds of success rate. The application of genomics refers to the analysis of human genetic material and aims to identify potential targets, making pharmaceutical drug discovery faster and cheaper.
In the year 2023, the incorporation of genomic testing market in relation to the discovery of drugs has enabled the understanding of more than 1500 new biomarkers leading to the creation of new targeted therapies. The use of genomic data in clinical trials has also increased and there are currently more than 800 trials employing genetic insight to categorize groups of patients. Such a method increases the effectiveness of new drugs as well as decreases side effects leading to the provision of better and improved drugs. It is noteworthy that over 200 drugs have been approved by the FDA for use that were created with the help of genomic assessment.
The annual investment in the development of drugs through genomic testing market has reached $8 billion, an amount which demonstrates a strong commitment from the public and private sectors. This development has already caused the establishment of more than 300 collaborations between pharmaceutical companies and genome research centers. Moreover, various technologies, such as CRISPR and AI data analysis, have increased the number of implemented projects with drug discoveries to over 400. The interest towards more personalized medicine that uses the data from genome sequencing to get the right treatment for the right genetic types has brought up to 100 personalized therapies which are being developed. All of these factors in one way or another increase the significance of genomic testing in the context of drug discovery and development, making it one of the key points in pharmacological studies of today.
By Technology
All stem cell therapy remains an important aspect of genomic testing market, and it occupied 42.4% share, which is supported by developments in regenerative medicine. Researchers across the world are currently exploring the potential applications of stem cells, with a stunning over 100 active clinical trials ongoing. As of 2023, there have been over a million people globally who have been injected with or have received stem-cell based treatments as an alternative, service, thus showing the growing acceptance and application of such treatments. Overall, It was reported that there were more than 500 centers for stem cell research activities across the world. When there was confidence in the safety and effectiveness of these therapies, more than 300 therapies based on stem cells were sanctioned in 2023.
The global ecosystem is witnessing a rapidly growing focus on CRISPR technology, project after project with unique implementation of modifying stem cells precision wise. The reach for tailored made stem cell therapies is marking a significant uptick since more than 700,000 patients have reported a need for this customized treatment. In the last one year, there have been more than 200 journal publications worldwide on stem cell therapy related research indicating continuous academic interest and research on the various possibilities of these therapies. Over 150 collaborations focused on furthering the field of stem cells and its applications have been forged between biotech companies and universities.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
To Understand More About this Research: Request A Free Sample
Regional Analysis
Currently, North America is still the leader in the global genomic testing market by accounting for 35% market share in 2023. The region enjoys good healthcare systems, innovative technological developments, and huge funding aimed at research and development activities in genomic research. It is also acknowledged that the region hosts some of the most advanced genomic labs and centers in the world; for instance, US alone has over 500 such centers. These centers in 2023 carried out over 2 million genomic tests, a considerable change in comparison to other years. Furthermore, efforts in the region, especially US government programs including All of Us Research Program supported by National Institutes of Health which seeks to collect genetic information from 1 million persons show the willingness to pursue better genomic exploration.
The advanced technological capacities of North America genomic testing market are also highlighted by its involvement in the development processes for next-generation sequencing (NGS) technologies. It started from intended efforts of companies located in the region, such as Illumina and Thermo Fisher Scientific, towards decreasing the price tag of the whole genome sequencing. The cost to sequence one whole human genome has been reduced to approximately $600 in 2023, from a $1,000 mark in the year 2020. This economic advantage has catalyzed the availability of genome tests and led to heightened integration of genomic testing into clinical work. Furthermore, North Americans researchers were among the first to come up with innovations such as CRISPR and its use for diagnostic purposes, which broadened the scope of genetic editing even further.
Genomic testing market has high demand and a strong understanding of it in North America. The number of individuals who bought the kits from direct-to-consumer genetic testing companies in North America reached over 20 million. In addition, the government allocated over $ 4 billion in the year 2023 to promoting initiatives in genomic research which indicates strong funding support. There are 100 more than healthcare systems in the North America, especially the US, that utilize genomic data in electronic health records to improve the quality of care offered to the patients. Such synergies of these elements account for a significant proportion of the North American market share which is estimated to be at 35% and explains its central importance in establishing and directing the momentum of the genomic testing market in the world.
Top Companies in Global Genomic Testing Market:
- Agilent Technologies, Inc.
- BGI Group
- Bio-Rad Laboratories
- Danaher Corporation
- Eurofins Genomics
- F. Hoffmann-La Roche
- Illumina, Inc.
- QIAGEN
- Singular Genomics Systems, Inc.
- Thermo Fisher Scientific, Inc.
- Other Prominent players
Market Segmentation Overview:
By Offering:
- System and Software
- Reagents & Consumables
- Services
- DTC Genomic Testing Service
- Sale of genomic data
By Testing Type:
- Sequencing Solution
- DNA Sequencing
- RNA Sequencing
- Methylation Sequencing
- NGS Library Preparation
- Sequencing Data Analysis
- Others
- Microarray Solution
- Human Genotyping with Arrays
- Non-Human Genotyping with Arrays
- Methylation Array Analysis
- Microarray Data Analysis
- Others
By Technology:
- Proteomics
- Pharmacogenomics
- Stem Cell Therapy
- Cloning
By Indication:
- Cancer
- Asthma
- Diabetes
- Hearth Diseases
- Other Indication
By Application:
- Drug Discovery & Development
- Disease Diagnosis
- Agricultural Production
- Others
By End-User:
- Hospitals & Clinics
- Research Centers & Academic Institutions
- Pharmaceutical & Biotechnology Companies
- Others
By Region:
- North America
- The U.S.
- Canada
- Mexico
- Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- Malaysia
- Thailand
- China
- India
- Japan
- Australia & New Zealand
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- Saudi Arabia
- South Africa
- UAE
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
REPORT SCOPE
| Report Attribute | Details |
|---|---|
| Market Size Value in 2023 | US$ 14.51 Billion |
| Expected Revenue in 2032 | US$ 32.95 Billion |
| Historic Data | 2019-2022 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Unit | Value (USD Bn) |
| CAGR | 9.54% |
| Segments covered | By Offering, By Testing Type, By Technology, By Indication, By Application, By End-User, By Region |
| Key Companies | Agilent Technologies, Inc., BGI Group, Bio-Rad Laboratories, Danaher Corporation, Eurofins Genomics, F. Hoffmann-La Roche, Illumina, Inc., QIAGEN, Singular Genomics Systems, Inc., Thermo Fisher Scientific, Inc., Other Prominent players |
| Customization Scope | Get your customized report as per your preference. Ask for customization |
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)