Europe In-Vitro Diagnostics Market: By Product and Services (Reagents, Instruments, Software, Services); Technique (Immunodiagnostics, Hematology, Molecular Diagnostics, Tissue Diagnostics, Clinical Chemistry, Others); Application (Cancer diagnostics, Blood glucose monitoring, Human genetic testing, Immunoassays, Hepatitis tests, Infectious Diseases diagnostics, Cardiac Diseases, Nephrological Diseases, Gastrointestinal Diseases, Others); End Users (Standalone Laboratories, Hospitals, Academic And Medical Schools, Point Of Care, Others); Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2024–2032
- Last Updated: 02-Apr-2024 | | Report ID: AA0923623
Market Scenario
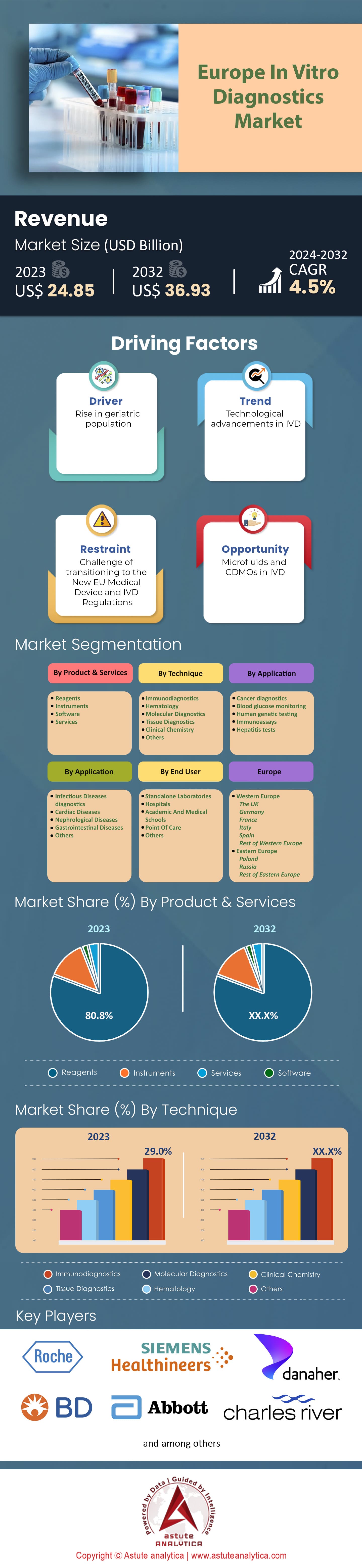
Europe in-vitro Diagnostics Market was valued at US$ 24.85 billion in 2023 and is projected to attain a market size of US$ 36.93 billion by 2032 at a CAGR of 4.5% during the forecast period 2024–2032.
Europe, a melting pot of culture, innovation, and medical advancements, houses one of the most lucrative markets for In Vitro Diagnostics (IVD) globally. Wherein, the United Kingdom, Germany, France, Italy, and Spain play central roles in the Europe in-vitro diagnostics market. Often labeled the "Big Five," they dominate the European IVD scene. These countries jointly account for approximately 70% of the total market share. Among these, Germany took the lead thanks to the thriving healthcare infrastructure of these nations, a steadily rising awareness of preventative medicine, and a robust regulatory framework that ensures the quality and efficacy of diagnostic tools.
At the heart of this growth is technology. Technological advancements act as the market's primary catalyst. With innovations like next-generation sequencing, Europe's IVD sector has witnessed an increased appetite for investment. In 2021, European ventures in this technology domain saw investments surpassing $500 million. Moreover, while still in its nascent stage, AI-driven diagnostics are capturing attention, having already garnered a $120 million investment. These figures hint at a future where technology will be intertwined with diagnostics, reshaping healthcare delivery and outcomes.
However, the COVID-19 pandemic left a significant impact on the Europe in-vitro diagnostics market. It served as a significant game-changer for the IVD industry in Europe. There was a remarkable year-on-year growth of 12% in 2021, primarily driven by the overwhelming global demand for COVID-19 testing kits. The European IVD sector produced over 300 million such kits that year, meeting almost 40% of the global demand. While this growth trajectory was commendable, the pandemic also showcased a noticeable decline of 7% in non-COVID-related diagnostics, emphasizing the market's shifting priorities during the crisis.
The In-vitro diagnostics market's competitive landscape is dynamic and ever-evolving. Recent years have seen Europe witnessing more than 25 major mergers and acquisitions in the IVD arena, indicating a trend towards market consolidation. Dominant players like Roche, Siemens, and Becton Dickinson collectively accounted for nearly 50% of the total market revenue in 2022. Yet, startups shouldn't be overlooked. They've carved their niche, with venture capital funding for IVD innovation in Europe hitting a record $800 million in 2022. Apart from this, an interesting trend shaping the future of Europe in-vitro diagnostics market is the patient-centric shift in healthcare delivery. This shift is best illustrated by the rising popularity of home-based IVD kits, whose sales surged by 18% in 2021, a trend accentuated by the pandemic's demand for remote healthcare solutions. Wearable diagnostics is another notable field to watch. With sales exceeding $100 million in 2022, marking a 20% rise from the previous year, wearable diagnostics represent a promising frontier for patient-centered care.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: The Aging European Demography and Chronic Disease Burden
Europe is home to one of the oldest populations globally. According to the World Health Organization, by 2050, approximately 40% of Europeans will be over 60 years old. This aging population is accompanied by an increasing prevalence of chronic diseases, such as diabetes, cardiovascular diseases, and cancers. The European Heart Network estimates that cardiovascular diseases alone account for over 3.9 million deaths annually in Europe, which is around 45% of all deaths.
Such health challenges necessitate early diagnosis, monitoring, and management, making IVD tools indispensable. IVD devices and services, which offer timely and accurate detection, are now more critical than ever. These diagnostics enable better patient management, guide treatment plans, and significantly reduce the associated healthcare costs. Thus, the growing geriatric populace coupled with the escalating chronic disease burden acts as a powerful driver for the In-vitro diagnostics market in Europe.
Trend: Personalized Medicine and Genomic Profiling
The age of "one-size-fits-all" medicine is slowly fading. Making waves in the European medical community is the shift towards personalized or precision medicine. Personalized medicine aims to tailor medical treatment to individual characteristics, needs, and preferences of each patient. At the heart of this trend lies the powerful tool of genomic profiling. According to the European Commission, over €1 billion has been invested in personalized medicine research through the Horizon 2020 programme. These investments are bearing fruit, especially in the in-vitro diagnostics market, where tests can now evaluate an individual's genetic makeup to determine susceptibility to certain diseases or predict therapeutic response. For instance, BRCA1 and BRCA2 gene mutation tests have become pivotal in assessing breast cancer risks and guiding treatment choices.
This growing reliance on genetic and molecular data, facilitated by IVDs, is setting a trend. Hospitals, clinics, and even direct-to-consumer businesses in Europe are adopting these advanced diagnostic tools. It's a paradigm shift, heralding a new era where treatments are as unique as the individuals receiving them.
Restraint: Regulatory Hurdles and Post-Market Scrutiny
While the European In-vitro diagnostics market has numerous tailwinds propelling it forward, it's not devoid of challenges. A significant restraint comes in the form of the intricate and often stringent regulatory landscape. The introduction of the EU's In-Vitro Diagnostic Regulation (IVDR) in 2017 has intensified the challenges for IVD stakeholders. Designed to enhance patient safety and ensure the reliability of diagnostic results, IVDR brought many changes, such as stricter post-market surveillance and reclassification of certain IVDs, leading to a more rigorous conformity assessment.
Although the intent behind IVDR is commendable, its implementation poses significant challenges for manufacturers. Smaller IVD firms, in particular, have expressed concerns over the increased time and financial resources required to navigate this regulatory maze. The BVMed, a German Medical Technology Association report, highlighted that almost 30% of smaller IVD manufacturers might consider exiting the in-vitro diagnostics market due to these regulatory challenges. Moreover, the post-market scrutiny, which mandates manufacturers to continuously monitor, report, and react to product performance in real-life scenarios, adds another layer of complexity. While this ensures enhanced patient safety, it also places additional pressures on manufacturers, potentially stymying innovation and market entry of novel diagnostics.
Segmental Analysis
By Product & Services:
By product and services, reagent segment is dominating the European in-vitro diagnostics market. Capturing an overwhelming market share of 80.8%, reagents undeniably form the backbone of the IVD industry in Europe. Their paramount importance is rooted in their role as essential components in diagnostic tests. Reagents are responsible for producing the actual reactions with specimens, leading to the visualization or quantification of results, making them indispensable for accurate diagnostics.
Given the rising demand for early and precise diagnosis in the in vitro diagnostics market, paired with the increased prevalence of chronic diseases in Europe's aging population, the demand for high-quality reagents has soared. The trend towards personalized medicine and precision diagnostics further fuels the need for specialized reagents, as these tests often rely on the specificity and sensitivity provided by them. Moreover, the continual advancements in diagnostic technology and the surge in research and development activities around IVDs have only bolstered the position of reagents in the market.
By Technique:
Based on techniques, European In-vitro diagnostics market is dominated by immunodiagnostics by holding an impressive revenue share of over 29%. This segment's dominance can be attributed to its wide array of applications, spanning from infectious disease detection to oncology. The reliability, cost-effectiveness, and relatively quick turnaround times offered by immunodiagnostics contribute to its reigning position.
However, while immunodiagnostics currently dominates the market, it's molecular diagnostics segment that show remarkable potential for the future. With projections indicating the highest Compound Annual Growth Rate (CAGR) of 4.9% in the segment, molecular diagnostics are swiftly ascending the ladder. Their rising prominence is anchored in their unparalleled precision and the capability to detect diseases at the molecular level. Whether it's diagnosing infectious diseases, genetic disorders, or predicting patient response to specific therapies, molecular diagnostics offer a level of granularity that's unparalleled. The burgeoning investments in genomic research and the accelerating shift towards personalized medicine in Europe further underscore the potential growth of this segment.
By Application:
The European In-vitro diagnostics market, when segmented by application, showcases the preeminence of infectious diseases diagnostics by commanding around 47.4% of the market share. This segment's dominance is attributed to Europe's focused efforts on early detection, management, and control of infectious diseases. Factors such as the recurrent outbreaks of infections, increased travel, and global interconnectedness, combined with heightened public awareness, propel the demand in this segment. The recent COVID-19 pandemic further amplified the indispensability of infectious diseases diagnostics, as rapid and accurate testing became a cornerstone for managing the crisis.
However, the in vitro diagnostics market horizon is looking particularly bright for immunoassays. Although they currently don't hold the largest market share, they're positioned to leapfrog with an impressive CAGR of 5.2%. Immunoassays, which primarily utilize antibodies to detect the presence of specific molecules, are witnessing increased adoption due to their sensitivity, scalability, and wide-ranging applications. From hormone level measurements to drug testing, the versatility of immunoassays is pushing their growth. The ongoing innovations in this space, coupled with a shift towards multiplexed detection, further fuel the potential rise of immunoassays in the European market.
By End Users:
Based on end-user aspect of the European In-vitro diagnostics market, hospitals emerge as the undeniable leaders, holding a substantial 41% revenue share. Hospitals, with their comprehensive infrastructural capabilities, are often the primary hubs for extensive diagnostic testing. The vast patient inflow, coupled with the need for timely and precise diagnostics for treatment decisions, places hospitals at the epicenter of the In-vitro diagnostics market. Their dominance is also bolstered by the centralized nature of healthcare in many European countries, where hospitals are primary care providers.
Yet, the limelight is gradually shifting towards point-of-care (POC) diagnostics. While they currently don't dominate the market, they're pegged to grow at a robust CAGR of 4.9%. POC diagnostics, characterized by their ability to deliver rapid results at or near the patient site, are revolutionizing the IVD landscape. The convenience, speed, and user-friendliness they offer make them particularly appealing, especially in outpatient settings, remote areas, and for home-based care. The COVID-19 pandemic, which necessitated decentralized and quick testing solutions, has undoubtedly catalyzed the momentum for POC diagnostics.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
To Understand More About this Research: Request A Free Sample
Regional analysis
Europe, a diverse tapestry of countries with varied healthcare systems and policies, presents a fascinating picture in the In-vitro diagnostics market. From the UK's emerging potential to Germany's steadfast leadership, the landscape is shaped by both historical investments and future-looking policies.
Starting with the UK, it stands out as a region poised for significant growth. With projections suggesting it will rise with the highest CAGR of 6.6%, the future of IVD in the UK appears particularly promising. This anticipated surge is backed by clear policy direction and intent. The recently published UK Life Sciences Vision underscores early diagnosis and treatment as pivotal missions. This, a collaborative effort between the government and the life science sector, outlines the country's aspirations to foster a burgeoning life sciences arena while addressing the major health challenges. Notably, while the UK's per capita spending on IVD testing has historically been on the lower side, the momentum is shifting. The burgeoning point of care services, coupled with a general uplift in the medical diagnostics sector, are primed to propel investments in IVD. It's evident that as healthcare expenditure in the UK tilts towards diagnostics, the In-vitro diagnostics market stands to benefit substantially.
Meanwhile, Germany firmly occupies the throne as a leader in the European In-vitro diagnostics market. This dominance is rooted in its consistent and significant investments in diagnostics. Channeling around 0.8% to 1% of its healthcare expenditure into IVD, Germany's allocation is unparalleled among its European counterparts. Such sustained investment not only anchors its leadership position but also serves as a testament to the country's belief in early and accurate diagnostics.
Trailing Germany, France occupies a formidable position in the market, solidifying the duo's dominance in the European landscape. However, not all countries boast such hefty market shares. Russia and Poland, for instance, find themselves on the opposite end of the spectrum, accounting for relatively minor shares. On the horizon, Spain and Italy are gearing up to become formidable players, with expected CAGRs of 4.9% and 3.6% respectively. Their growth trajectories hint at upcoming policies, investments, and infrastructural changes that are likely to favor the In-vitro diagnostics market.
Top Players in Europe In-Vitro Diagnostics Market
- Abbott
- Agilent Technologies, Inc.
- Becton Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories, Inc.
- Charles River Laboratories
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- Qiagen
- Quest Diagnostics
- Quidel Corp.
- Siemens Healthineers
- Sysmex Corp.
- Other Prominent Players
Market Segmentation Overview:
By Product & Services
- Reagents
- Instruments
- Software
- Services
By Technique
- Immunodiagnostics
- Hematology
- Molecular Diagnostics
- Tissue Diagnostics
- Clinical Chemistry
- Others
By Application
- Cancer diagnostics
- Blood glucose monitoring
- Human genetic testing
- Immunoassays
- Hepatitis tests
- Infectious Diseases diagnostics
- Cardiac Diseases
- Nephrological Diseases
- Gastrointestinal Diseases
- Others
By End User
- Standalone Laboratories
- Hospitals
- Academic And Medical Schools
- Point Of Care
- Others
By Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
REPORT SCOPE
| Report Attribute | Details |
|---|---|
| Market Size Value in 2023 | US$ 24.85 Bn |
| Expected Revenue in 2032 | US$ 36.93 Bn |
| Historic Data | 2019-2022 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Unit | Value (USD Bn) |
| CAGR | 4.5% |
| Segments covered | By Product & Services, By Technique, By Application, By End User |
| Key Companies | Abbott, Agilent Technologies, Inc., Becton Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories, Inc., Charles River Laboratories, Danaher Corporation, F. Hoffmann-La Roche Ltd., Qiagen, Quest Diagnostics, Quidel Corp., Siemens Healthineers, Sysmex Corp., Other Prominent Players |
| Customization Scope | Get your customized report as per your preference. Ask for customization |
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)