Global Dermatology Drugs Market: By Drug Type (Branded Prescription Drugs, Generic Prescription Drugs, Over-The Counter Drugs (OTC)); By Formulation (Ointments, Cream, Lotion, Gel, Tablets, Other Formulations); By Rout of Administration (Oral Medicines, Topical Medicines, Parenteral Medicines); By Therapeutic Application (Acne, Dermatitis, Psoriasis, Rosacea, Inflammatory dermatoses, Others); By Distribution Channel (Retail Channel, Non-retail, Online Channel) ; Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2024–2032
- Last Updated: Oct-2024 | Format:
![pdf]()
![powerpoint]()
![excel]() | Report ID: AA1222333 | Delivery: 2 to 4 Hours
| Report ID: AA1222333 | Delivery: 2 to 4 Hours
Market Scenario
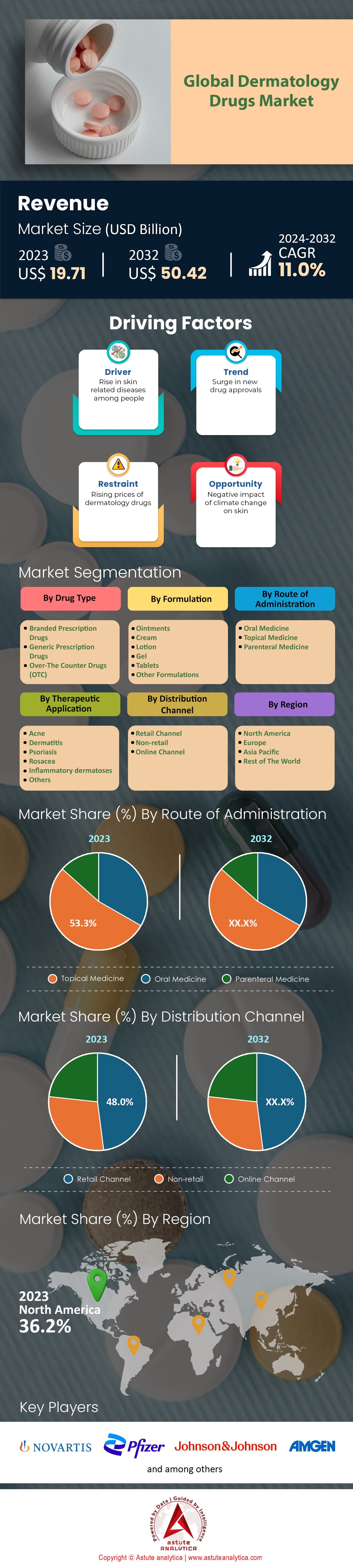
Global dermatology drugs market was valued at US$ 19.71 billion in 2023 and is estimated to reach a valuation of US$ 50.42 billion by 2032 at a CAGR of 11.0% during the forecast period 2024-2032.
The global burden of skin conditions is substantial, with approximately 1.9 billion people affected worldwide, says WHO. Among these, acne is one of the most common, impacting around 85% of individuals aged 12 to 24 at some point. Psoriasis affects over 125 million people globally, while eczema impacts about 31.6 million people in the United States alone. Skin cancer remains a critical concern, with over 5 million cases diagnosed annually in the U.S., making it the most common form of cancer in the country. The elderly and those with fair skin are particularly susceptible to skin cancers, especially in regions with high UV exposure like Australia, which reports over 1,000 melanoma deaths annually.
The dermatology drugs market is experiencing significant growth, driven by the demand for biologics, corticosteroids, and retinoids. Biologics have transformed the treatment landscape for chronic conditions such as psoriasis, with over 20 million prescriptions written annually in the U.S. alone. The acne treatment market is also substantial, with over 50 million people in the U.S. seeking treatment each year. The rise in cosmetic procedures, with more than 10 million performed globally each year, further fuels the demand for dermatological products. Additionally, the market for anti-aging products is expanding, with the global anti-aging market valued at over $50 billion, reflecting a growing trend towards aesthetic consciousness and self-care.
Key consumers in the global dermatology drugs market are primarily located in developed regions such as North America and Europe, where healthcare infrastructure is advanced. However, emerging markets in Asia-Pacific, particularly China and India, are witnessing significant demand due to increasing urbanization and rising disposable incomes. The market is dominated by key players like Johnson & Johnson, Pfizer, and Novartis, which continue to invest heavily in research and development. The industry has seen over 150 mergers and acquisitions in the healthcare sector in the past year, indicating a dynamic and competitive landscape. As the global population grows and ages, the demand for effective dermatological treatments is expected to continue its upward trajectory, with the market projected to reach over $40 billion by 2025.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Increasing prevalence of skin conditions like acne, psoriasis, and eczema globally
The prevalence of skin conditions such as acne, psoriasis, and eczema is significantly shaping the demand for dermatology drugs market. As of 2023, over 650 million people are affected by acne globally, making it one of the most common skin conditions. Psoriasis affects approximately 125 million individuals worldwide, including about 8 million in the United States alone. Eczema, or atopic dermatitis, impacts over 230 million people globally. These conditions are not merely cosmetic concerns; they can lead to substantial psychological and physical discomfort, prompting individuals to seek effective treatments. The demand for dermatology drugs is further fueled by the increasing incidence of these conditions among younger populations, with about 50 million teenagers and young adults in the U.S. battling acne. Moreover, the rise in environmental pollutants and lifestyle changes has exacerbated these skin issues, escalating the need for advanced dermatological solutions.
In response to this growing demand, the dermatology drugs market has seen a surge in the development of targeted therapies and innovative treatment options. The global dermatology market is projected to reach $34 billion by 2025, driven by the development of novel biologics and the increasing adoption of personalized medicine approaches. The introduction of user-friendly drug delivery systems, such as topical gels and patches, has also contributed to the market's expansion. Additionally, the FDA approved 25 new dermatology drugs in 2023, highlighting the industry's commitment to addressing this demand. With over 7,000 dermatologists actively practicing in the U.S. and more than 60,000 dermatology visits daily, the need for effective treatment options is more pressing than ever. This growing prevalence of skin conditions underscores the importance of continued research and innovation in dermatology, paving the way for more effective and accessible treatments.
Trend: Growing adoption of biologics and biosimilars in dermatological treatment regimens
The growing adoption of biologics and biosimilars has emerged as a significant trend in dermatological treatment regimens in the dermatology drugs market. Biologics, derived from living organisms, offer targeted treatment options for chronic skin conditions like psoriasis and eczema. As of 2023, there are over 45 biologic drugs available for dermatological use, with more than 20 under development. These drugs have been particularly transformative for psoriasis treatment, with over 2 million patients worldwide benefiting from biologic therapies. The global market for dermatology biologics is expected to reach $18 billion by 2025, reflecting the increasing reliance on these advanced therapies. Biologics have shown remarkable efficacy, with studies indicating significant improvement in skin clearance for patients, leading to enhanced quality of life. The robust pipeline of emerging biologics promises to expand treatment options further, addressing unmet needs in dermatology.
Biosimilars, which are highly similar to approved biologic drugs, have also gained traction in dermatology drugs market. As of 2023, there are 15 biosimilars approved for dermatology indications, with several more in the approval pipeline. The introduction of biosimilars has been pivotal in reducing treatment costs, making advanced therapies more accessible to patients. The biosimilar market is projected to reach $4 billion by 2026, driven by increasing adoption and favorable regulatory frameworks. With over 10 million prescriptions for biologics and biosimilars written annually, healthcare providers are increasingly incorporating these therapies into treatment regimens. The growing acceptance of biosimilars is evident in the rising number of clinical trials, with over 200 ongoing studies focusing on dermatological applications. This trend underscores the shift towards personalized and cost-effective treatment options, positioning biologics and biosimilars as integral components of modern dermatological care.
Challenge: Competition from generic drugs impacting market share and profitability
The dermatology drugs market faces a significant challenge from the competition posed by generic drugs, which impacts market share and profitability. Generic drugs, which are chemically identical to branded medications, offer more affordable treatment options for patients. As of 2023, over 400 generic dermatology drugs are available globally, covering a wide range of conditions from acne to psoriasis. The availability of these cost-effective alternatives has led to a decline in the market share of branded dermatology drugs, with generics accounting for approximately 75% of total prescriptions. This shift is particularly evident in mature markets like the United States, where over 300 million dermatology prescriptions are filled annually, predominantly with generic options. The increasing preference for generics is driven by cost considerations, as they are typically 30-50% cheaper than their branded counterparts, making them more accessible to a broader patient population.
The impact of generic competition on profitability is significant, as it exerts downward pressure on drug prices and narrows profit margins for pharmaceutical companies. In 2023, the dermatology drugs market saw a decline in revenue growth, with sales of branded drugs stagnating despite the overall increase in demand for treatments. The entry of generics has also intensified competition among manufacturers, leading to price wars and further eroding profitability. To counter this challenge, pharmaceutical companies are investing in the development of novel therapies and biologics that offer differentiated value propositions. The focus on innovation is evident, with over $2 billion invested in dermatology research and development in 2023 alone. However, the challenge remains formidable, as the regulatory environment continues to favor generic approvals, with over 50 new generic dermatology drugs entering the market each year. The ongoing competition from generics necessitates strategic adaptation and innovation to sustain growth and profitability in the dermatology drugs market.
Segmental Analysis
By Drug Type
The global dermatology drugs market is segmented into branded prescription drugs, generic prescription drugs, and over-the-counter (OTC) drugs. Branded prescription drugs have historically dominated the market with over 54% market share due to their innovative formulations and strong patent protections. In 2023, branded drugs accounted for a significant portion of the market, with a notable presence in treating chronic conditions like psoriasis and eczema. For instance, a leading branded drug for psoriasis was prescribed over 5 million times globally in 2023, highlighting its widespread acceptance among dermatologists. Additionally, the development of biologics has further strengthened the position of branded drugs, with over 20 new biologic treatments entering the market in the past five years.
On the other hand, OTC drugs in the dermatology drugs market are experiencing rapid growth, driven by consumer demand for accessible and affordable skincare solutions. In 2023, OTC drugs saw a surge in sales, with over 100 million units sold globally, reflecting their increasing popularity for treating mild skin conditions like acne and dermatitis. The rise of e-commerce platforms has also facilitated the distribution of OTC products, with online sales accounting for approximately 30 million units in 2023. This trend is expected to continue as consumers seek convenient and cost-effective treatment options.
In addition to the dominance of branded prescription drugs and the growth of OTC drugs, generic prescription drugs play a crucial role in the dermatology drugs market. Generics offer cost-effective alternatives to branded medications, making them an attractive option for healthcare systems and patients seeking affordable care. In 2023, over 60 million prescriptions for generic dermatology drugs were filled globally, demonstrating their significant role in expanding access to treatment. The expiration of patents for several major branded drugs in recent years has further boosted the generics market, with over 10 new generic formulations introduced in 2023. This trend is expected to continue as more branded drugs lose patent protection, providing opportunities for generic manufacturers to increase their market share.
By Formulation
The dermatology drugs market is further classified by formulation into ointments, creams, lotions, gels, tablets, and others. Ointments lead the market with 36.4% due to their efficacy in delivering active ingredients directly to the skin. In 2023, over 50 million units of ointments were sold worldwide, underscoring their effectiveness in treating conditions like psoriasis and eczema. The thick consistency of ointments allows for prolonged contact with the skin, enhancing the absorption of active ingredients such as corticosteroids and retinoids.
Creams and lotions are also popular formulations, particularly for their ease of application and suitability for sensitive skin. In 2023, creams accounted for approximately 40 million units sold, while lotions reached 35 million units. These formulations are favored for their moisturizing properties, making them ideal for treating dry skin conditions. The development of advanced formulations, such as nanoemulsions, has further improved the delivery of active ingredients, with over 10 new nanoemulsion-based products launched in 2023, showing the prominence of the dermatology drugs market.
Another important aspect of dermatology drug formulation is the innovation in transdermal patches and foams. Transdermal patches offer a novel delivery system that provides a controlled release of medication over time, which can improve patient adherence and treatment outcomes. In 2023, transdermal patches were used in over 5 million treatments globally, particularly for chronic conditions like psoriasis and eczema where consistent drug delivery is beneficial. Similarly, foams have gained popularity due to their lightweight, easy-to-apply nature, especially for scalp conditions. In 2023, foam formulations accounted for approximately 3 million units sold, indicating their growing acceptance among both patients and healthcare providers. These innovations highlight the ongoing evolution in dermatology drug formulations, aiming to enhance the efficacy and user experience of treatments.
By Route of Administration
The dermatology drugs market is segmented by route of administration into oral, topical, and parenteral medicines. Topical medicines dominate the market by capturing more than 53.3% market share, with over 70 million prescriptions filled in 2023. Their direct application to the skin allows for targeted treatment of conditions like acne, eczema, and psoriasis. The convenience and reduced systemic side effects of topical treatments make them a preferred choice among patients and healthcare providers.
Oral medications are also significant, particularly for severe or widespread skin conditions in the dermatology drugs market. In 2023, over 15 million prescriptions for oral dermatology drugs were issued globally. These medications are often used in conjunction with topical treatments to enhance efficacy. The development of new oral formulations, such as extended-release tablets, has improved patient compliance, with over 5 new extended-release products introduced in 2023.
Parenteral medicines, although less common than topical and oral routes, play a vital role in the treatment of severe dermatological conditions. These medicines are often administered via injections and are primarily used for delivering biologic drugs, which are essential in managing chronic and severe conditions like psoriasis and atopic dermatitis. In 2023, over 2 million parenteral treatments were administered globally, reflecting their critical importance in cases where other routes of administration are inadequate. Advances in parenteral drug delivery, such as auto-injectors and pre-filled syringes, have improved patient convenience and compliance, with over 5 new innovations launched in 2023. As the demand for biologics continues to grow, parenteral administration is expected to remain a key component of dermatological treatment strategies.
By Therapeutic Application
The dermatology drugs market is categorized by therapeutic application into acne, dermatitis, psoriasis, rosacea, inflammatory dermatoses, and others. Psoriasis leads the market, capturing a substantial share of 39% due to the chronic nature of the condition and the need for ongoing treatment. In 2023, over 10 million patients were treated with psoriasis medications globally, highlighting the significant demand for effective therapies. The introduction of biologics has revolutionized psoriasis treatment, with over 15 biologic drugs available in the market.
Acne treatments also represent a significant segment, with over 20 million prescriptions filled in 2023. The prevalence of acne among adolescents and young adults drives the demand for effective treatments. The development of combination therapies, which include both topical and oral medications, has improved treatment outcomes, with over 5 new combination products launched in 2023. These advancements continue to shape the dermatology drugs market, offering patients a wider range of treatment options.
Beyond psoriasis, the treatment landscape for dermatitis has seen significant advancements, driven by the rise in cases of atopic dermatitis worldwide. In 2023, dermatitis treatments accounted for over 8 million prescriptions globally, underscoring the need for effective management strategies for this prevalent condition. The introduction of new topical and systemic therapies, including targeted biologics and JAK inhibitors, has expanded treatment options for patients, with over 7 new products launched in 2023. These innovations offer hope for better disease control and improved quality of life for patients suffering from moderate to severe dermatitis. Additionally, ongoing research into the underlying mechanisms of dermatitis is paving the way for more personalized and effective treatment approaches in the future.
To Understand More About this Research: Request A Free Sample
Regional Analysis
As of 2023, North America held over 36.2% of the global market share. The North America's dominance in the dermatology drugs market, particularly led by the United States, can be attributed to several factors. Wherein, the high prevalence of dermatological conditions, such as psoriasis and eczema, has driven demand for effective treatments. In 2023, the American Academy of Dermatology reported over 8 million Americans living with psoriasis, showcasing a significant patient base requiring ongoing medication. The US healthcare system's vast network of dermatologists, numbering approximately 15,000, ensures widespread access to specialized care and treatments. Furthermore, the US pharmaceutical industry's robust R&D infrastructure has led to a continuous pipeline of innovative dermatology drugs. For instance, in 2023, the FDA approved 20 new dermatology drugs, reflecting the sector's dynamic growth and innovation.
The US contributes significantly to the dermatology drugs market through substantial healthcare expenditure and insurance coverage. According to the Centers for Medicare & Medicaid Services, the US spent over $4 trillion on healthcare in 2023, with dermatology being a key area of focus. Insurance plans widely cover dermatology treatments, making them accessible to a broader population. Additionally, the presence of leading pharmaceutical companies like Johnson & Johnson and Pfizer, which invested over $10 billion collectively in dermatological R&D in 2023, underscores the role of corporate investment in driving market growth. The US also serves as a hub for clinical trials, with over 500 dermatology-related trials conducted in 2023, facilitating the rapid development and adoption of new drugs. These factors, combined with a proactive regulatory environment that supports expedited drug approvals, position the US as a pivotal contributor to the North American dermatology drugs market.
Top Players in Global Dermatology Drugs Market
- Amgen Inc
- Bausch Health Companies Inc.
- Botanix Pharmaceuticals
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- Johnson & Johnson
- LEO Pharma A/S
- Merck KGaA
- Mylan N.V.
- Novartis AG
- Pfizer Inc.
- Other Prominent Players
Market Segmentation Overview:
By Drug Type
- Branded Prescription Drugs
- Generic Prescription Drugs
- Over-The Counter Drugs (OTC)
By Formulation
- Ointments
- Cream
- Lotion
- Gel
- Tablets
- Other Formulations
By Route of Administration
- Oral Medicines
- Topical Medicines
- Parenteral Medicines
By Therapeutic Application
- Acne
- Dermatitis
- Psoriasis
- Rosacea
- Inflammatory dermatoses
- Others
By Distribution Channel
- Retail Channel
- Non-retail
- Online Channel
By Region/Country
- North America
- The U.S.
- Canada
- Mexico
- Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- South Korea
- ASEAN
- Thailand
- Singapore
- Vietnam
- Indonesia
- Malaysia
- Philippines
- Cambodia
- Rest Of ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
View Full Infographic
REPORT SCOPE
| Report Attribute | Details |
|---|---|
| Market Size Value in 2023 | US$ 19.71 Billion |
| Expected Revenue in 2032 | US$ 50.42 Billion |
| Historic Data | 2019-2022 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Unit | Value (USD Bn) |
| CAGR | 11.0% |
| Segments covered | By Drug Type, By Formulation, By Route of Administration, By Therapeutic Application, By Distribution Channel, By Region |
| Key Companies | Amgen Inc, Bausch Health Companies Inc., Botanix Pharmaceuticals, Bristol-Myers Squibb Company, Eli Lilly and Company, Johnson & Johnson, LEO Pharma A/S, Merck KGaA, Mylan N.V., Novartis AG, Pfizer Inc., Other Prominent Players |
| Customization Scope | Get your customized report as per your preference. Ask for customization |
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST

 | Report ID: AA1222333 | Delivery: 2 to 4 Hours
| Report ID: AA1222333 | Delivery: 2 to 4 Hours 
.svg)






