Global Clinical Trials Support Service Market: By Trial Type (Centralized Clinical Trial and Decentralized Clinical Trial); Therapeutic Type (Small molecules, Biologic drugs, Medical Therapeutic Types); Phases (Preclinical Studies, Phase I, Phase II, Phase III, and Phase IV); Services (Site Management, Patient Recruitment, Clinical Trial management, Data Management, Others); Application (Oncology, CNS and mental disorders, Cardiovascular diseases, Infectious diseases, Blood disorders, Others); End Users (Pharmaceutical & Biotechnology companies, Contract Research Organizations (CROs), Medical Therapeutic Types Companies, Others); Regional— Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2024–2032
- Last Updated: Feb-2024 | Format:
![pdf]()
![powerpoint]()
![excel]() | Report ID: AA0923622 | Delivery: 2 to 4 Hours
| Report ID: AA0923622 | Delivery: 2 to 4 Hours
Market Scenario
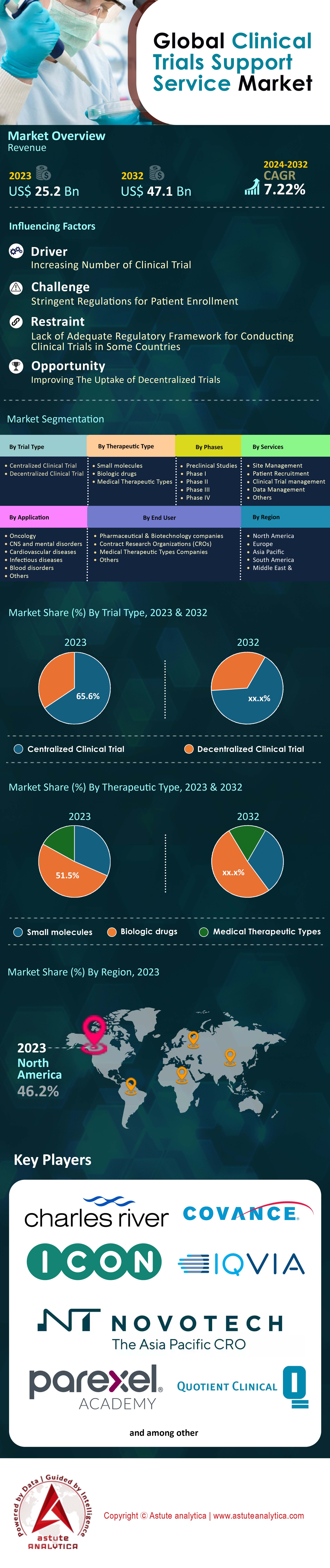
Global Clinical Trials Support Service Market was valued at US$ 25.2 billion in 2023 and is projected to surpass US$ 47.1 billion by 2032 at a CAGR of 7.22% during the forecast period 2024–2032.
The clinical trials support service market is an essential backbone to the global healthcare industry. It plays a pivotal role in ushering in innovative treatments and breakthrough therapeutics. As the world witnesses a surge in various diseases and conditions, there's an increased emphasis on developing newer, safer, and more effective treatments. This drive has significantly bolstered the demand for robust and streamlined clinical trial support services. The CDC, in its recent publications, highlighted the surge in clinical trials, especially in the domain of infectious diseases. This surge can be attributed to the recent global health challenges that necessitated rapid research and solution development. The focus isn't just on drug development but also on understanding the long-term effects and possible complications of diseases, which further fuels the demand for extended clinical trial support.
The FDA, another crucial body governing drug approvals and clinical trials in the U.S., reported an increase of 15% in the number of new drug application (NDA) submissions in the past year. This rise, while indicative of the thriving pharmaceutical industry, also underlines the growing need for clinical trial support services. With each new drug or therapeutic application, there's a vast amount of data to be processed, managed, and analyzed, making the role of these support services indispensable.
A noteworthy trend in the clinical trials support service market is the shift toward patient-centric trials. The FDA has been emphasizing the importance of patient-reported outcomes, which adds another layer of complexity to the trials. Services that can aid in patient recruitment, retention, and real-world data collection are gaining prominence. This evolution has led to a spike in demand for specialized support services, further propelling the market forward. Regionally, North America, primarily the U.S., remains a significant player in the clinical trials market. However, Asia-Pacific, led by countries like China and India, is fast emerging as a hub for clinical research. This shift is driven by the combination of skilled labor, advanced infrastructure, and favorable regulatory environments. As per the latest reports, Asia-Pacific witnessed a growth rate of 9% in clinical trials, outpacing the global average.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Technological Advancements in Data Analytics and Artificial Intelligence
The integration of advanced data analytics and artificial intelligence (AI) has become a notable driver propelling the clinical trials support service market forward. Historically, clinical trials have been rooted in manual procedures, but the present scenario has witnessed significant technological advancements. A single phase III clinical trial, for instance, was estimated to produce upwards of 3 terabytes of data in 2022. With projections suggesting a 10% annual increase in such data generation by 2025, the sheer volume becomes overwhelming.
Addressing this burgeoning data, tools powered by AI and advanced analytics have been transformative. By 2025, it's anticipated that over 65% of clinical trials support services will employ AI or similar advanced analytics in their operations. These aren't just technological boons; they have financial implications as well. The potential cost savings from implementing AI tools in drug development processes can range between 15-20%. Given that the average cost to develop a new prescription drug that gains clinical trials support service market approval hovered around $2.6 billion in 2021, the cost-saving potential of AI is in the billions.
Moreover, AI-powered predictive analytics has changed the dynamics of decision-making in drug development. Previously, deciding on the feasibility of a drug pathway took months. But with the advent of AI, such decisions are now being made in weeks or sometimes even days. A study showcased that nearly 70% of pharmaceutical companies utilizing AI managed to cut their decision-making timelines by half.
Trend: Increased Emphasis on Patient Diversity in Clinical Trials
A prominent trend gaining momentum in the clinical trials support service market is the increasing emphasis on patient diversity. A few years ago, diversity in clinical trials was not a primary focus. However, in 2019, only about 20% of clinical trials reported having a diverse patient base. By 2022, this figure leaped to 35%. The trajectory suggests that by 2025, we might see over half of the new clinical trials placing a priority on patient diversity. Regulatory bodies have significantly influenced this trend. For instance, when the FDA released guidelines in 2020 emphasizing the need for patient diversity in clinical trials, it created ripples in the industry. Post these guidelines, about 40% of pharmaceutical companies reported revising their recruitment strategies to ensure a broader and diverse participant base.
Diversity in clinical trials isn't only a matter of representation; it's an economically sound strategy. Drugs tested across a more varied patient base are likely to face fewer hurdles in regulatory approval processes, which translates to faster market access in the clinical trials support service market. Estimates indicate that companies emphasizing diverse trials can speed up their drug's time-to-market by up to six months. This can potentially increase their revenues by 10-15%. Further, focusing on diversity has another intangible yet profound impact: building trust. Historically underrepresented communities have often been skeptical about clinical research. However, a 2022 survey highlighted that 60% of respondents from these communities would be more willing to participate in clinical trials if they were aware of the trial's commitment to diversity.
Challenge: Data Privacy and Security Concerns in Clinical Trials
In the global clinical trials support service market, one challenge stands out prominently: ensuring data privacy and security. As clinical trials have progressively shifted towards digital platforms, the vast amounts of sensitive patient data generated are at an increased risk of breaches and unauthorized access. The transition to electronic health records and digital data collection tools, while enhancing efficiency, has amplified the vulnerability of the system. In 2021, for instance, there was a reported 30% increase in cyber-attacks targeting healthcare data, compared to the previous year. Such attacks not only jeopardize the integrity of the trial but also risk the privacy of participants, potentially exposing their personal health information.
The global nature of many clinical trials also introduces another layer of complexity. Different countries have their own sets of regulations and standards concerning data privacy. Navigating these varied regulatory landscapes can be challenging for pharmaceutical companies and clinical trial organizers. A study in 2022 found that nearly 40% of international clinical trials faced regulatory hurdles due to discrepancies in data privacy standards between countries. Wherein, the ramifications of these challenges are manifold. Trust, being the cornerstone of patient participation in clinical trials, is at stake. If participants feel their data might be at risk, they are less likely to enroll, which can impede the progress of potentially life-saving research. In an era defined by digital transformation, ensuring robust data privacy and security remains a critical challenge for the global clinical trials support service market.
Segmental Analysis
By Trial Type:
Based on trial type, the centralized clinical trial segment stands out prominently in the global clinical trials support service market. Accounting for a substantial 65.6% of the market share, it has established a clear dominance in the landscape. Historically, centralized clinical trials have always played a pivotal role in the research domain, providing advantages in terms of standardized protocols, uniform data collection methods, and streamlined communication channels.
The centralized clinical trial segment is also projected to grow at the fastest CAGR of 7.41% over the forecast period. If these projections hold true, the segment's market share could potentially inch closer to 70% by the end of the decade, further cementing its leadership position. The accelerated growth can be attributed to the increasing complexity of trials, the rise in multi-regional clinical trials, and the advantages centralized trials offer in terms of cost-effectiveness and time efficiency.
By Therapeutic Type:
On the therapeutic front, the market exhibits a distinct tilt towards biologics drugs. Capturing an impressive 51.5% of the market share, biologics drugs have become the mainstay in the global clinical trials support service market. Biologics, given their intricate nature and potential for treating a wide range of diseases, from autoimmune disorders to cancers, have seen a surge in research interest and investment.
Furthermore, the trend doesn't seem to be plateauing anytime soon. Biologics drugs, mirroring the growth pattern seen in the centralized clinical trial segment, are expected to climb at the highest CAGR of 7.41% in the upcoming years. This consistent growth in the biologics segment is propelled by a myriad of factors. The increasing understanding of genetics, the rise in personalized medicine, and the continuous advancements in biotechnology are just a few catalysts pushing biologics to the forefront of therapeutic research.
By Phases:
By trial phases, Phase III emerges as the most dominant in the global clinical trials support service market, accounting for a commanding 48% of the market share. This significant dominance can be attributed to the critical nature of Phase III trials in the drug development pipeline. These trials, often termed as pivotal studies, involve a larger cohort of participants and are instrumental in assessing the efficacy and potential side effects of the treatment in question. Given the scale and complexity of Phase III trials, they require extensive resources, monitoring, and management, thereby driving a considerable portion of support services.
Another factor bolstering the dominance of Phase III is the regulatory importance it holds. Successful completion of this phase often becomes the cornerstone for regulatory approval from bodies like the FDA or EMA. With drug developers aiming to ensure their investigative treatments clear this phase with definitive positive outcomes, investments in support services for Phase III are naturally high.
To Understand More About this Research: Request A Free Sample
By Services:
Shifting focus to services, the clinical trial management segment stands out in the global clinical trials support service market, capturing an impressive 54% of the market share. Clinical trial management encompasses a range of essential services, from patient recruitment, site selection, and data management to regulatory compliance and reporting. Given the intricate nature of modern clinical trials, with their multifaceted protocols, diverse patient populations, and stringent regulatory requirements, effective management becomes imperative.
The predominance of the clinical trial management segment can also be attributed to the increasing globalization of clinical trials. With studies being conducted across multiple sites, often in different countries or continents, there's a pronounced need for centralized management to ensure uniformity and adherence to standards. This demand for seamless, coordinated management across geographically dispersed sites naturally funnels investments and reliance into clinical trial management services.
Moreover, as the industry gravitates towards patient-centric approaches and real-world data, the nuances of clinical trial management have expanded, further emphasizing the importance of this service segment in the global clinical trials support service market. Ensuring patient compliance, integrating real-world data, and leveraging digital tools are now integral parts of trial management, necessitating specialized support services.
Regional Analysis
North America stands as the undeniable leader in the clinical trials support service market, commanding an impressive 46.2% revenue share. The region's dominance is largely attributed to the United States, which has historically been a nexus for groundbreaking medical research and innovation. Housing a significant number of globally recognized research institutions and pharmaceutical companies, the region witnesses a significant influx of financial investments in clinical research. For instance, in 2021 alone, the National Institutes of Health (NIH) committed over $41 billion towards medical research. This robust research infrastructure, coupled with stringent regulatory requirements of the Food and Drug Administration (FDA), necessitates a vast array of specialized support services to ensure compliance, efficiency, and quality in clinical trials. Furthermore, the rise of novel therapeutic areas, such as precision medicine and genetic therapies, has increased the number of clinical trials in the region by an estimated 8% year-on-year, further fueling the demand for support services.
Europe clinical trials support service market, on the other hand, holds its own as a significant player, capturing over 26% of the market share in the clinical trials support service domain. The European landscape is enriched by its diverse demographics and patient populations, offering a unique advantage for clinical trials aiming for more generalized results. In the financial year 2021-2022, countries like Germany, France, and the UK saw a combined investment of €15 billion in clinical research, indicating the region's commitment to advancing medical science. Europe's attractiveness is further enhanced by the European Medicines Agency (EMA) and its consistent efforts towards regulatory harmonization across member states. Such initiatives have significantly reduced the bureaucratic hurdles once associated with multi-country trials in the region. As a result, the demand for clinical trial support services in Europe has seen a consistent annual growth of around 6%.
Top Players in the Global Clinical Trials Support Service Market
- Charles River Laboratories
- Covance Inc.
- Icon PLC
- inVentiv International Pharma Services
- IQVIA
- Laboratory Corp. of America Holdings
- Novotech
- Parexel International
- Pharmaceutical Product Development LLC
- Quotient Bioresearch
- WuXi AppTec
- Leukemia & Lymphoma Society (LLS)
- Other prominent players
Market Segmentation Overview:
By Trial Type
- Centralized Clinical Trial
- Decentralized Clinical Trial
By Therapeutic Type
- Small molecules
- Biologic drugs
- Medical Therapeutic Types
By Phases
- Preclinical Studies
- Phase I
- Phase II
- Phase III
- Phase IV
By Services
- Site Management
- Patient Recruitment
- Clinical Trial management
- Data Management
- Others
By Application
- Oncology
- CNS and mental disorders
- Cardiovascular diseases
- Infectious diseases
- Blood disorders
- Others
By End User
- Pharmaceutical & Biotechnology companies
- Contract Research Organizations (CROs)
- Medical Therapeutic Types Companies
- Others
By Region
- North America
- The US
- Canada
- Mexico
- Europe
- The U.K.
- Germany
- France
- Spain
- Poland
- Belgium
- Finland
- Netherlands
- Portugal
- Sweden
- Switzerland
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- ASEAN
- South Korea
- Rest of Asia Pacific
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Morocco
- Rest of MEA
- South America
- Brazil
- Argentina
- Colombia
- Chile
- Peru
- Rest of South America
View Full Infographic
REPORT SCOPE
| Report Attribute | Details |
|---|---|
| Market Size Value in 2023 | US$ 25.2 Billion |
| Expected Revenue in 2032 | US$ 47.1 Billion |
| Historic Data | 2019-2022 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Unit | Value (USD Bn) |
| CAGR | 7.22% |
| Segments covered | By Trial Type, By Therapeutic Type, By Phases, By Services, By Application, By End User, By Region |
| Key Companies | Charles River Laboratories, Covance Inc., Icon PLC, inVentiv International Pharma Services, IQVIA, Laboratory Corp. of America Holdings, Novotech, Parexel International, Pharmaceutical Product Development LLC, Quotient Bioresearch, WuXi AppTec, Leukemia & Lymphoma Society (LLS), Other prominent players |
| Customization Scope | Get your customized report as per your preference. Ask for customization |
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST

 | Report ID: AA0923622 | Delivery: 2 to 4 Hours
| Report ID: AA0923622 | Delivery: 2 to 4 Hours 
.svg)






